Abstract
OBJECTIVES:
To identify BMI trajectories utilizing methods and graphing tools that maintain and visualize variability of BMIs ≥95th percentile, and to investigate individual differences in early sociodemographic risk, infant growth and feeding patterns, and maternal weight status on these trajectories.
STUDY DESIGN:
Participants included 1,041 predominantly rural, poor families from the Family Life Project, a longitudinal birth cohort. Youth anthropometrics were measured 8 times between ages 2 months and 12 years; at 2 months, mothers reported sociodemographics, youth birthweight, infant feeding, and self-reported child weight and height (at 2 months and 12 y). At 6 months, mothers reported breastfeeding. At 2 years maternal weight and height were measured.
RESULTS:
Three BMI trajectories were identified: “maintained non-overweight,” “developed obesity,” and “developed severe obesity.” Compared with the non-overweight trajectory, the children with heavier trajectories were breastfed for a shorter duration and had heavier mothers at all assessments. The children with “developed obesity” trajectory were not heavier at birth than those with non-overweight trajectory, yet, they displayed greater change in weight-for-length percentile during infancy; additionally, their mothers had the highest change in BMIs between 2 months and 12y. Children with the “developed severe obesity” trajectory was heavier at birth, and more likely to be heavy during infancy and fed solid foods early.
CONCLUSIONS:
Using informed analytical and graphing approaches, we described patterns of growth, and identified early predictors of obesity and severe obesity trajectories among a diverse sample of poor, rural youth. Researchers are urged to consider these approaches in future work, and to focus on identifying protective factors in youth with obesity and severe obesity.
Keywords: Childhood obesity, infant growth, sociodemographics, maternal weight status
Pediatric obesity and severe obesity continue to be major health problems in the United States (1, 2). Pediatric obesity is defined as having a body mass index (BMI; m2/kg) ≥ 95th percentile, and severe obesity as having a BMI ≥120% of the 95th percentile for age and sex (2). Based on recent national data, ~18.5% of youth have obesity, and 5.6% have severe obesity (3); higher rates are reported among minority and rural youth (2, 4). Youth with obesity are at greater risk for chronic comorbidities including type 2 diabetes and metabolic syndrome (5, 6); far worse cardiometabolic risk profiles have been reported for youth with severe obesity.
Despite the numerous studies on child growth, our knowledge is limited regarding trajectories of high BMI growth (≥95th percentile) in non-clinical settings, particularly in regards to severe obesity and its early life precursors (eg, 7, 8–11). This may in part be due to the frequent use of BMI percentiles and z-scores to study child growth (e.g., 12, 13). These indices are compressed at their highest values and can result in children with obesity and severe obesity being lumped together (14), despite emerging evidence for their distinct cardiometabolic risk (15), adrenal dysfunction (16), and disordered eating behaviors (16). Additionally, the use of BMI z-scores may lead to misleading findings in children with high BMI in longitudinal research (18), given the compression of variability in BMI > 95th percentile. Solutions to this problem have been proposed. Some authors recommend using raw BMI scores in longitudinal data analyses instead of BMI percentiles or z-scores (19, 20). Adjusted WHO BMI-z scores specifically avoid compression in the tails of the distribution by expressing z-scores < −3 or > 3 in units of the distance between ±2 SD and ±3 SD (21), respectively, and are widely used for longitudinal tracking. Another problem has been the lack of available graphing tools that include severe obesity growth curves and are appropriate for longitudinal research; the absence of these tools has made it difficult to visualize the substantial variability in BMI ≥95th percentile (see (22) for an illustration). Lastly, among the few studies that have examined BMI trajectories ≥95th percentile (9, 23), growth mixture modeling techniques—a method for identifying unobserved sub-populations in a dataset (24)—were not employed, which may have resulted in limited conclusions about the development of obesity and severe obesity.
We utilized growth mixture modeling to identify BMI trajectories between ages 2 to 12 years in a representative, longitudinal sample of rural, poor youth using methods that maintain and visualize variability ≥95th BMI percentile. In doing so, we demonstrate the utility of a BMI-for-age graphing tool (22), designed to include severe obesity curves. Given the potential risk for obesity conferred by infant growth and feeding patterns (25–29), early sociodemographic risk (26–27), and maternal weight status (26, 30), we also examined these factors as predictors of BMI trajectories.
METHODS
Data were drawn from the Family Life Project (31), a longitudinal, birth cohort study of predominantly poor youth and families residing in rural communities in the United States. Extensive study details are provided at https://flp.fpg.unc.edu. Pregnant, English-speaking mothers residing in six poor, rural counties in North Carolina and Pennsylvania were recruited at birth in 2003; low-income and Black families were oversampled. A total of 1,292 youth were followed from birth to 12 years; home visits occurred at ages 2 and 6 months, and 2, 3, 4, 5, 7, and 12 years. Because we were interested in modeling normative BMI growth patterns, we excluded youth with <2 BMI data points between 2 to 12 y (n=157) and with low birthweights (<2500g; n=94). This resulted in a final sample of 1041 youth. This study was approved by the Institutional Review Boards at the sponsoring universities for the North Carolina and Pennsylvania sites.
Measures
Youth BMI.
Youth anthropometrics were collected by trained data collectors using standardized procedures. At 2 and 6 months, infant weight (to the nearest 0.01 kg) and length (0.1 cm) were measured, and used to compute weight-for-length (WFL) and BMI percentiles based on WHO recommendations (21). Primary caregivers were asked to remove all of their youth’s clothing, except for a dry diaper, and to place the infant in a recumbent position, faced up on the scale. Measurements were taken once. Change scores between 2 to 6 months were computed for WFL and BMI percentiles, separately. From age 2 to 12 years, standing height (0.1 cm) and weight (0.1 kg) (shoes and heavy clothing removed) were measured once, and used to compute CDC age and sex-specific BMI scores and percentiles (32). CDC reference criteria were used to define non-overweight (BMI <85th percentile), overweight (BMI ≥ 85th percentile), and 3 categories of obesity: class I obesity as BMI ≥95th percentile, class II obesity (severe obesity) as BMI ≥120% of the 95th percentile, and class III (severe obesity) as BMI ≥140% of the 95th percentile. Seven youth had one implausible BMI data point (±3 standard deviations from the mean) that was inconsistent with their overall BMI trajectory, suggesting that the data points were erroneous; these specific data points were removed.
Predictors.
At the 2-month visit, mothers were asked to recall their infant’s birthweight in pounds and ounces, which was converted to grams. At 2 months, mothers reported if their infant was fed solid foods (e.g., cereal) in the past week (1=yes, 0 = no); at 6 months, mothers reported if their infant was ever breastfed or fed breast milk, and how long their youth was breastfed thus far; responses were coded into number of weeks. At 2 y, mothers height and weight were measured by a trained research assistant; all measurements were taken once. Mothers self-reported their height and weight at 2 and 6 months, and 12 y. These data were used to compute BMI scores, and weight classifications for obesity class I (BMI of 30 to < 35.0), class II (BMI of 35 to < 40.0) and class III (BMI ≥ 40; severe obesity).
At the 2-month visit, mothers reported on familial sociodemographics, including family structure (single- vs. two-parent), household income, and maternal education. Household income was determined based on all earners residing in the home, and was summed across household residents. Individuals were considered a household resident if he/she spent 3+ nights/week in the home. Income-to-needs ratio (INR) was calculated using 2004 poverty threshold values. These sociodemographic variables will be referred to as sociodemographic risk henceforth. Youth sex and race were collected at recruitment.
Statistical Analyses
To identify BMI trajectories, growth mixture modeling (GMM) was performed separately for boys and girls in Mplus 8.0 (Muthen & Muthen, Los Angeles, CA); missing data were handled using full information maximum likelihood (FIML; 33). As suggested in (19, 20), raw BMI scores were used to generate BMI trajectories. Additionally, by using raw BMI scores, we were able to employ the BMI-for-age graphing tool published by Racette et al for visualizing wide variability in growth.(22) First, unconditional latent growth curve models were fit to determine if BMI change from 2 to 12y was linear, quadratic, and/or cubic. Only the linear and quadratic growth terms were statistically significant at P < 0.05; thus, the intercept, linear, and quadratic growth terms were included in the following mixture models. Second, quadratic latent class growth models (LCGMs)—a simplified version of GMM in which the variance and covariance of the growth measures were constrained to zero—, estimating one to ten classes, were performed as a starting point in class identification. Next, GMM models were run with the within-class variance of the intercept and slope allowed to vary; 1 to 5 classes were estimated. Model fit was assessed using the Bayesian Information Criterion (BIC; 34), Lo-Mendell-Rubin Likelihood Ratio Test (LMR-LRT; 35), and boostrap likelihood ratio test (BLRT; 36). We also evaluated entropy, shape of the BMI trajectories, interpretability, and latent class size. Steps were taken to ensure that the best-fitting models were not local solutions (33). Estimated means were outputted using the PLOT command and entered into the SAS macro syntax published by Racette, Yu, DuPont, and Clark (22) to generate sex-specific BMI-for-age graphs that displayed our trajectories. These graphs were developed, based on CDC youth reference datasets and weight status criteria, to accommodate a wide BMI range and include severe obesity curves. Small changes were made to the macro to adjust figure formatting.
A total of 25 multiple imputations were performed in SAS 9.4 (SAS Institute Inc., Cary, NC, USA) (37). All study variables, including BMI trajectory membership, were included to inform the imputation. Only 6.6% of data points were missing across the entire sample; levels of missingness were similar across the BMI trajectory groups (5.5 to 6.6%). Table I lists nonimputed and imputed descriptive statistics. All descriptives and multivariate modeling estimates were combined using PROC MI ANALYZE. Multinomial logistic regression was used to evaluate if sociodemographic risk, infancy growth and feeding patterns, and maternal BMI predict BMI trajectory membership.
Table 1 (Online).
Distribution of study variables among imputed and nonimputed samples
| Imputed data set descriptives | Nonimputed data set descriptives | ||
|---|---|---|---|
| Mean % (95% CI) | Mean % (95% CI) | N (sample size of available data) | |
| Youth and sociodemographic risk characteristics | |||
| Youth sex, % male | 51.5 (48.5 to 54.5) | 51.5 (48.5 to 54.5) | 1041 |
| Youth race, % African American | 42.0 (39.0 to 45.0) | 42.0 (39.0 to 45.0) | 1041 |
| Family structure, % single-parent | 66.4 (63.6 to 69.3) | 66.4 (63.6 to 69.3) | 1041 |
| Household income-to-needs ratio | 1.9 (1.7 to 2.0) | 1.9 (1.7 to 2.0) | 1015 |
| Maternal education, years | 12.7 (12.6 to 12.9) | 12.7 (12.6 to 12.9) | 1041 |
| State of residence, % living in North Carolina | 58.6 (55.6 to 61.6) | 58.6 (55.6 to 61.6) | 1041 |
| Infant growth | |||
| Birthweight, grams | 3381 (3353 to 3409) | 3381 (3353 to 3409) | 1041 |
| BMI, 2 months | 16.3 (16.2 to 16.4) | 16.3 (16.2 to 16.4) | 1019 |
| BMI, 6 months | 17.9 (17.8 to 18.0) | 17.9 (17.8 to 18.0) | 1004 |
| BMI change score, 2 to 6 months | 1.5 (1.4 to 1.6) | 1.5 (1.4 to 1.7) | 982 |
| WFL, 2 months | 54.4 (52.4 to 56.3) | 54.3 (52.4 to 56.3) | 1019 |
| WFL, 6 months | 65.2 (63.5 to 66.9) | 65.1 (63.3 to 66.8) | 1004 |
| WFL change score, 2 to 6 months | 10.8 (8.7 to 12.8) | 10.9 (8.8 to 12.9) | 982 |
| Infant dietary patterns assessed at 6 months | |||
| Breastfed,% | |||
| Ever been breastfed | 47.7 (44.1 to 50.2) | 47.5 (44.4 to 50.6) | 1010 |
| Breastfeeding, duration (wks) | 7.2 (6.5 to 7.8) | 7.3 (6.6 to 7.9) | 1010 |
| Consumed solid foods in the past week, 2 months % | 42.1 (39.1 to 45.1) | 98.5 (97.7 to 99.2) | 1011 |
| Maternal BMI | |||
| 2 months | 28.4 (28.0 to 28.8) | 28.2 (27.8 to 28.6) | 976 |
| 6 months | 29.5 (29.0 to 30.0) | 29.1 (28.6 to 29.5) | 962 |
| 24 months | 29.6 (29.1 to 30.1) | 29.1 (28.7 to 29.6) | 910 |
| 12 years | 32.1 (31.5 to 32.7) | 32.1 (31.4) | 593 |
| 2 months to 12 y | 3.7 (3.2 to 4.1) | 3.7 (3.2 to 4.1) | 554 |
CI, confidence interval; INR, income-to-needs ratio; BMI, body-mass-index; WFL, weight-for-length.
All sociodemographic variables (i.e. household INR, family structure, and maternal education) were collected via maternal self-report when youth were 2 months of age.
RESULTS
Distribution of the study variables are presented in Table 2. At 2 y, 16.8% of youth (95% CI: 13.9 to 19.5) were classified with non-overweight, 12.4% (95% CI: 9.3 to 14.1) had class I obesity, and 9.1% (95% CI: 2.1 to 16.0) had severe obesity (class II and III).
Table 2.
Distribution of sociodemographic risk, infancy growth, and parental weight indicators for the total sample and by BMI trajectory membership
| Total Sample | “Maintained non-overweight” | “Developed obesity” | “Developed severe obesity” | |
|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| N, % | 1041, 100.0% | 797, 76.6% | 186, 17.9% | 58, 5.6% |
| Youth and sociodemographic risk characteristics 1 | ||||
| Youth sex, % | ||||
| Male | 51.5 | 50.8 | 60.2 | 32.8 |
| Female | 48.5 | 49.2 | 39.8 | 67.2 |
| Youth race, % | ||||
| White | 58.0 | 60.6 | 46.8 | 58.6 |
| African American | 42.0 | 39.4 | 53.2 | 41.4 |
| Family structure, % | ||||
| Single-parent | 66.5 | 67.6 | 61.8 | 65.5 |
| Two-parent | 33.5 | 32.4 | 38.2 | 34.5 |
| Household INR | 1.8 (1.7 to 2.0) | 1.9 (1.7 to 2.0) | 1.8 (1.5 to 2.0) | 2.0 (1.4 to 2.6) |
| Maternal education, years | 12.7 (12.6 to 12.9) | 12.8 (12.6 to 12.9) | 12.6 (12.3 to 12.9) | 12.7 (12.3 to 13.2) |
| State of Residence, % | ||||
| Pennsylvania | 41.4 | 43.0 | 37.6 | 31.0 |
| North Carolina | 58.6 | 57.0 | 62.4 | 69.0 |
| Infant growth | ||||
| Birthweight, grams | 3381 (3353 to 3409) | 3366 (3335 to 3397) | 3410 (3338 to 3482) | 3489 (3357 to 3621) |
| BMI, 2 months | 16.3 (16.2 to 16.4) | 16.2 (16.1 to 16.4) | 16.5 (16.3 to 16.8) | 17.0 (16.6 to 17.5) |
| BMI, 6 months | 17.9 (17.8 to 18.0) | 17.7 (17.6 to 17.9) | 18.2 (17.9 to 18.5) | 18.7 (18.1 to 19.2) |
| BMI change score, 2 to 6 months | 1.5 (1.4 to 1.6) | 1.5 (1.4 to 1.6) | 1.7 (1.4 to 2.0) | 1.6 (1.1 to 2.2) |
| WFL percentile, 2 months | 54.4 (52.4 to 56.3) | 53.1 (50.8 to 55.5) | 55.9 (51.1 to 60.7) | 66.2 (58.8 to 73.6) |
| WFL percentile, 6 months | 65.2 (63.5 to 66.9) | 62.8 (60.7 to 64.9) | 71.2 (66.8 to 75.6) | 78.3 (71.5 to 85.2) |
| WFL percentile change score, 2 to 6 months | 10.8 (8.7 to 12.8) | 9.7 (7.2 to 12.1) | 15.3 (10.0 to 20.6) | 12.2 (4.2 to 20.1) |
| Infant dietary patterns | ||||
| Consumed solid foods in the past week, 2 months % | 42.1 (39.1 to 45.1) | 40.0 (36.6 to 43.4) | 46.8 (39.6 to 53.9) | 55.2 (42.4 to 68.0) |
| Breastfeeding history, 6 months | ||||
| Ever been breastfed, % | 47.7 (44.1 to 50.2) | 48.8 (45.3 to 52.4) | 41.2 (34.0 to 48.4) | 43.6 (30.7 to 56.4) |
| Breastfeeding, duration (wks) | 7.2 (6.5 to 7.8) | 7.7 (7.0 to 8.5) | 5.7 (4.3 to 7.2) | 4.7 (2.3 to 6.8) |
| Maternal BMI | ||||
| 2 months | 28.4 (28.0 to 28.8) | 27.3 (26.9 to 27.7) | 31.6 (30.5 to 32.7) | 33.4 (31.2 to 35.7) |
| 6 months | 29.5 (29.0 to 30.0) | 28.2 (27.7 to 28.8) | 33.1 (31.8 to 34.4) | 35.2 (32.7 to 37.8) |
| 24 months | 29.6 (29.1 to 30.1) | 28.3 (27.8 to 28.8) | 33.3 (32.0 to 34.7) | 35.3 (32.8 to 37.7) |
| 12 years | 32.1 (31.5 to 32.7) | 30.8 (30.1 to 31.5) | 36.4 (34.9 to 37.9) | 35.8 (33.2 to 38.3) |
| 2 months to 12y | 3.7 (3.2 to 4.1) | 3.5 (−3.68, 10.7) | 4.8 (−5.1 to 14.6) | 2.4 (−2.6 to 7.4) |
CI, confidence interval; INR, income-to-needs ratio; BMI, body-mass-index; WFL, weight-for-length.
All sociodemographic variables (i.e. household INR, family structure, and maternal education) were collected via maternal self-report when youth were 2 months of age.
Youth BMI Trajectories
Boys’ and girls’ GMM model fit statistics are provided in Table 3. For boys, the BIC was lowest for the three class model; the LMR-LRT and BLRT both indicated that the 3-class model was a significant improvement over a model with 1 less class; and lastly, entropy values for the 3-class model were adequate at .77 and higher than the other classes. Based on this information and model interpretability, a 3-class model was selected for boys. Girls’ fit indices followed a very similar pattern (Table 3); thus, a 3-class model was selected for girls’ data.
Table 3 (Online).
Model fit statistics from growth mixture models (GMM)
| Youth Sex | Latent classes | BIC | LMR-LRT | BLRT | Convergence | Entropy | Log Likelihood | Size (n) of smallest class |
|---|---|---|---|---|---|---|---|---|
| Boys | 1 | 10476 | N/A | N/A | Yes | N/A | −5200.5 | 536 (100.0%) |
| 2 | 10105 | 0.026 | 0.000 | Yes | 0.69 | 101 (18.8%) | ||
| 3 | 10004 | 0.007 | 0.000 | Yes | 0.77 | −4930.1 | 19 (3.5%) | |
| 4 | 10012 | 0.343 | 0.073 | Yes | 0.72 | −4915.3 | 17 (3.2%) | |
| 5 | 10014 | 0.088 | 0.120 | Yes | 0.72 | −4897.5 | 16 (3.0%) | |
| Girls | 1 | 10184 | N/A | N/A | Yes | N/A | −6239.4 | 505 (100%) |
| 2 | 9883 | 0.005 | 0.000 | Yes | 0.68 | −5603.8 | 108 (21.4%) | |
| 3 | 9813 | 0.026 | 0.000 | Yes | 0.77 | −5317.8 | 39 (7.7%) | |
| 4 | 9822 | 0.242 | 0.235 | Yes | 0.63 | −5167.3 | 30 (5.9%) | |
| 5 | 9836 | 0.558 | 0.666 | Yes | 0.70 | −5058.9 | 15 (3.0%) |
BMI trajectories are presented in Figure 1, A and B. A separate set of plots that provide 95% confidence intervals are provided in Figure 2, A and B. As shown in Figure 1, A and B, very similar trajectories emerged for boys and girls; thus, their results were combined. Youth in the “maintained non-overweight” trajectory (75.6% of boys; 77.6% of girls) had mean BMI scores within the non-overweight range at all time points. Youth in the “developed obesity” trajectory (20.9% of boys; 14.7% of girls) had mean BMI scores exceeding the cut-off for obesity class I at 5y and older. Youth in the “developed severe obesity” trajectory (3.5% of boys; 7.7% of girls) had mean BMI scores that exceeded the cut-off for obesity class II (severe obesity) at 5y and followed an upward, accelerated trend towards obesity class III (severe obesity) between 5 and 12y. Distribution of study variables by BMI trajectory are presented in Table 2.
Figures 1a and 1b (ONLINE).
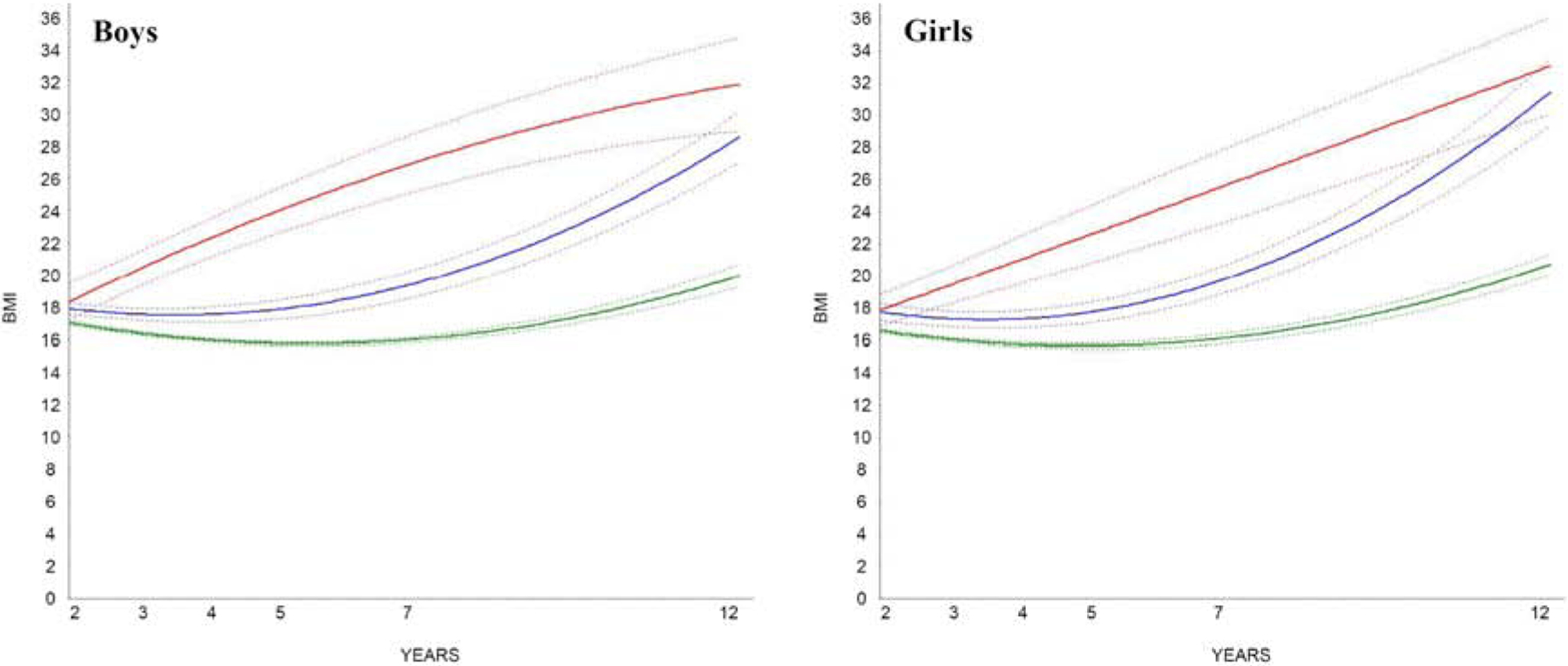
Growth mixture model solutions identifying BMI trajectories for boys and girls. Models were performed separately for boys and girls. Plotted solid lines represents estimated means; dashed lines are 95% confidence intervals.
 “Maintained Non-Overweight” (n=405, 75.6%) “Maintained Non-Overweight” (n=405, 75.6%) |
 “Maintained Non-Overweight” (n=392, 77.6%) “Maintained Non-Overweight” (n=392, 77.6%) |
 “Developed Obesity” (n=112, 20.9%) “Developed Obesity” (n=112, 20.9%) |
 “Developed Obesity” (n=74, 14.7%) “Developed Obesity” (n=74, 14.7%) |
 “Developed Severe Obesity” (n=19, 3.5%) “Developed Severe Obesity” (n=19, 3.5%) |
 “Developed Severe Obesity” (n=39, 7.7%) “Developed Severe Obesity” (n=39, 7.7%) |
FIGURE 2a and 2b.
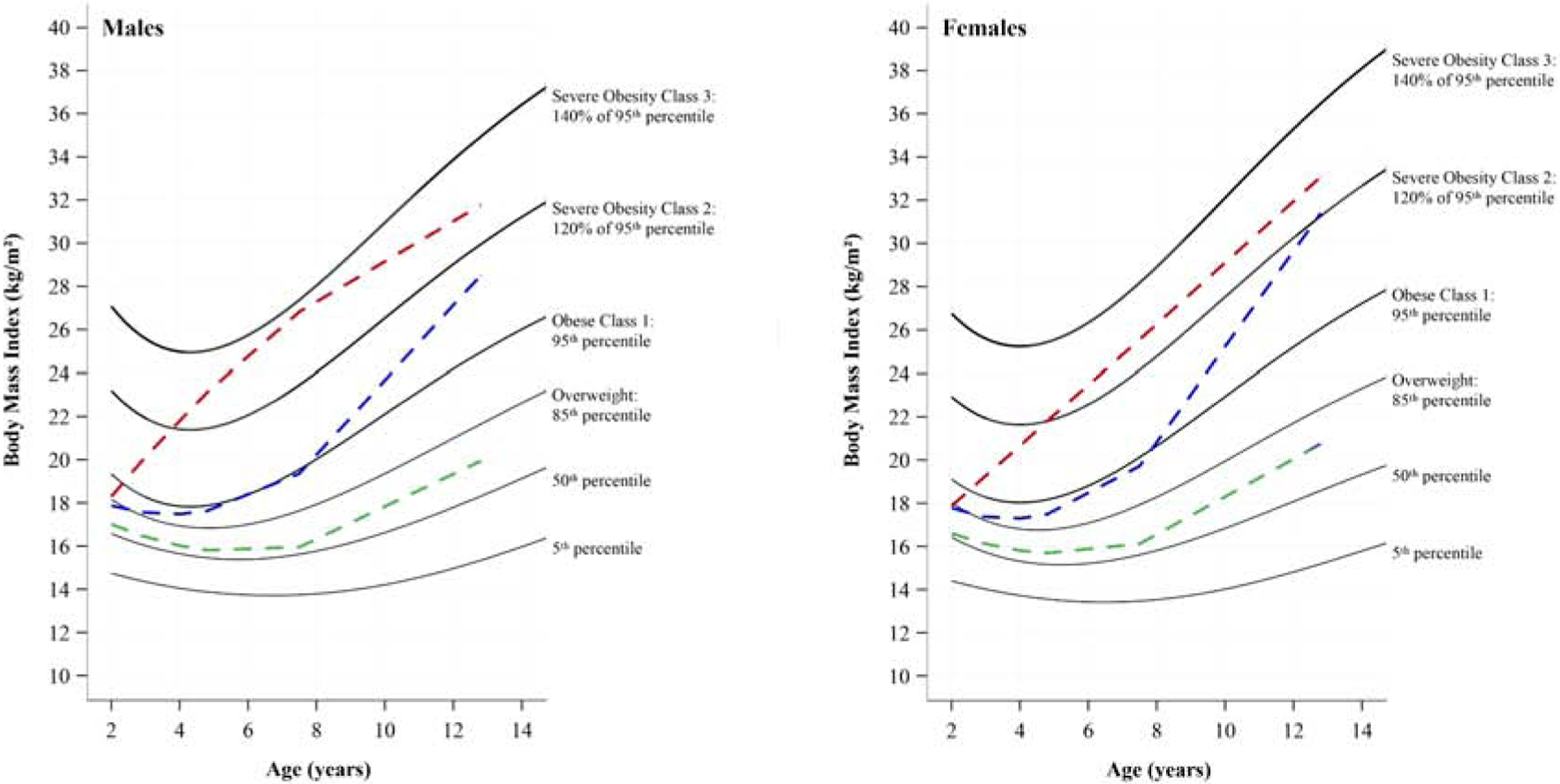
Latent class growth modeling solution of BMI trajectories for boys (a) and girls (b) plotted on sex-specific BMI-for-age graphs (19) designed to include severe obesity percentile curves, ages 2 to 12 y. BMI weight classifications cut-offs (i.e., solid lines) were determined based on CDC recommendations for overweight (BMI ≥ 85th percentile) and 3 categories of obesity: class I obesity as ≥95th BMI percentile, class II obesity (severe obesity) as ≥120% of the 95th BMI percentile, and class III (severe obesity) as ≥140% of the 95th percentile.
 “Maintained Non-Overweight” (n=405, 75.6%) “Maintained Non-Overweight” (n=405, 75.6%) |
 “Maintained Non-Overweight” (n=392, 77.6%) “Maintained Non-Overweight” (n=392, 77.6%) |
 “Developed Obesity” (n=112, 20.9%) “Developed Obesity” (n=112, 20.9%) |
 “Developed Obesity” (n=74, 14.7%) “Developed Obesity” (n=74, 14.7%) |
 “Developed Severe Obesity” (n=19, 3.5%) “Developed Severe Obesity” (n=19, 3.5%) |
 “Developed Severe Obesity” (n=39, 7.7%) “Developed Severe Obesity” (n=39, 7.7%) |
Youth Characteristics and Sociodemographic Risk
As shown in Table 4, youth in the “developed obesity” trajectory had greater odds of being male than the other two classes, and of being black than the “maintained non-overweight” trajectory. Youth in the “developed severe obesity” trajectory had reduced odds of being male than the “maintained non-overweight” trajectory. No other sociodemographic risk variables predicted BMI trajectory membership.
Table 4.
Odds ratios (ORs) of infancy growth and dietary indicators predicting youth BMI trajectory membership
| “Maintained non-overweight” | “Developed obesity” | “Developed severe obesity” | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| “Maintained non-overweight” BMI trajectory as reference group | |||
| Youth and sociodemographic risk characteristics | |||
| Youth male | REF | 1.46 (1.06, 2.03)* | 0.47 (0.27, 0.83)** |
| Youth black | REF | 1.75 (1.27, 2.41)*** | 1.09 (0.63, 1.87) |
| Family structure2 | REF | 0.78 (0.56, 1.08) | 0.91 (0.52, 1.59) |
| Household INR | REF | 0.96 (0.87, 1.03) | 1.05 (0.91, 1.20) |
| Maternal education, years | REF | 0.95 (0.88, 1.03) | 0.99 (0.87, 1.13) |
| State of Residence4 | REF | 1.25 (0.90, 1.74) | 1.68 (0.95, 2.98) |
| Infant growth | |||
| Birthweight, grams | REF | 1.0002 (0.9999, 1.0006) | 1.0006 (1.0001, 1.0013)* |
| BMI, 2 months | REF | 1.10 (1.01, 1.21)* | 1.32 (1.12, 1.54)*** |
| BMI, 6 months | REF | 1.17 (1.07, 1.28)*** | 1.33 (1.16, 1.54)*** |
| BMI change score, 2 to 6 months | REF | 1.06 (0.97, 1.16) | 1.05 (0.91, 1.21) |
| WFL percentile, 2 months | REF | 1.00 (1.00, 1.01) | 1.01 (1.01, 1.02)** |
| WFL percentile, 6 months | REF | 1.01 (1.01, 1.02)*** | 1.02 (1.01, 1.04)*** |
| WFL percentile change score, 2 to 6 months | REF | 1.01 (1.01, 1.01)* | 1.00 (0.99, 1.01) |
| Infant dietary patterns | |||
| Consumed solid foods in the past week, 2 months | REF | 1.32 (0.96, 1.82) | 1.84 (1.08, 3.15)* |
| Breastfeeding history, 6 months | |||
| Ever been breastfed | REF | 0.73 (0.53, 1.02) | 0.81 (0.47, 1.39) |
| Breastfeeding, duration (wks) | REF | 0.98 (0.96, 0.99)* | 0.97 (0.94, 0.99)* |
| Maternal BMI | |||
| 2 months | REF | 1.10 (1.07, 1.13)*** | 1.13 (1.09, 1.17)*** |
| 6 months | REF | 1.08 (1.06, 1.11)*** | 1.11 (1.08, 1.15)*** |
| 24 months | REF | 1.08 (1.06, 1.11)*** | 1.11 (1.07, 1.15)*** |
| 12 years | REF | 1.08 (1.06, 1.11)*** | 1.08 (1.04, 1.11)*** |
| 2 months to 12 years | REF | 1.05 (1.01, 1.09)* | 0.96 (0.91, 1.02) |
| “Obesity” BMI trajectory as reference group | |||
| Youth and sociodemographic risk characteristics 1 | |||
| Youth male | REF | 0.68 (0.49, 0.94)*** | |
| Youth black | REF | 0.62 (0.34, 1.13) | |
| Family structure2 | REF | 1.17 (0.63, 2.17) | |
| Household INR | REF | 1.09 (0.93, 1.28) | |
| Maternal education, years | REF | 1.04 (0.90, 1.20) | |
| State of Residence4 | REF | 1.34 (0.71, 2.52) | |
| Infant growth | |||
| Birthweight, grams | REF | 1.0004 (1.0000, 1.0007) | |
| BMI, 2 months | REF | 1.19 (1.01, 1.42)* | |
| BMI, 6 months | REF | 1.14 (0.98, 1.34) | |
| BMI change score, 2 to 6 months | REF | 0.99 (0.84, 1.15) | |
| WFL percentile, 2 months | REF | 1.01 (1.01, 1.02)* | |
| WFL percentile, 6 months | REF | 1.01 (0.99, 1.03)* | |
| WFL percentile change score, 2 to 6 months | REF | 1.00 (0.99, 1.01) | |
| Infant dietary patterns assessed at 6 months | |||
| Consumed solid foods in the past week, 2 months | REF | 1.40 (0.77, 2.53) | |
| Breastfeeding history, 6 months | |||
| Ever been breastfed | REF | 1.10 (0.61, 2.01) | |
| Breastfeeding, duration (wks) | REF | 0.90 (0.95, 1.02) | |
| Maternal weight | |||
| 2 months | REF | 1.03 (0.99, 1.07) | |
| 6 months | REF | 1.03 (0.99, 1.06) | |
| 24 months | REF | 1.02 (0.99, 1.06) | |
| 12 years | REF | 0.99 (0.96, 1.06) | |
| 2 months to 12 years | REF | 0.89 (0.93, 0.85)** | |
CI, confidence interval; BMI, body-mass-index; WFL, weight-for-length; REF, reference.
P < 0.05,
P < 0.01,
P < 0.001.
Infant Growth and Feeding
As shown in Table 4, youth with higher birthweights had greater odds of being in the “developed severe obesity” trajectory, compared with the “maintained non-overweight” trajectory, and the “developed obesity” group fell in-between. Youth with higher BMIs at 2 and 6 months had greater odds of being in the “developed obesity” and “developed severe obesity” trajectories than the “maintained non-overweight” trajectory, and youth with higher BMIs at 6 months had greater odds of ending up in the “developed severe obesity” trajectory than the “developed obesity” trajectory. Youth who were breastfed for a longer duration had reduced odds of being in the “developed obesity” and “developed severe obesity” trajectories, than the “maintained non-overweight” trajectory. Lastly, youth who were introduced to solid foods by 2 months were at increased odds of being in the “developed severe obesity” trajectory than in the “maintained non-overweight” trajectory.
Maternal BMI
As shown in Table 4, mothers with higher BMIs at all time points had greater odds of their youth being in the “developed obesity” and “developed severe obesity” trajectories than the “maintained non-overweight” trajectory. Mothers with the greatest change in BMI between 2 months and 12 years, had increased odds of their youth being in the “developed obesity” than the other two trajectories. Of note, at 24 months, 30.1% of youth (95% CI: 17.9 to 42.3) in the “developed severe obesity” trajectory had mothers with severe obesity, followed by 20.9% of youth (95% CI: 15.0 to 26.9) in the “developed obesity” trajectory, and 6.8% of youth (95% CI: 5.0 to 8.6) in the “maintained non-overweight. By 12 years, 33.3% of youth (95% CI: 25.9 to 40.7) in the “developed obesity” trajectory had mothers with severe obesity, followed by 26.9% of youth (95% CI: 14.6 to 39.2) in the “developed severe obesity” trajectory, and 12.3% of youth (95% CI: 9.5 to 15.1) in the “maintained non-overweight (data not shown).
DISCUSSION
In past studies, researchers have often used compressed BMI indices (e.g. BMI z-scores) and conventional modeling techniques (e.g. change scores) to study growth patterns, and/or did not have access to graphing tools (22) that accommodate high BMI growth and are appropriate for longitudinal, epidemiologic research. These methods may have masked trajectories of severe obesity in previous samples, lumping together obese and severely obese children, and may have contributed to a lack of understanding about the etiology of severe obesity. Given our findings, clinicians and researchers should consider the graphing tool published by Racette et al, along with published modified clinical growth charts (22)(38). (22) If children with obesity and severe obesity have unique BMI trajectories as early as 6 months old, visualizing and identifying early growth patterns will aid in early detection and inform intervention efforts aiming to alter excessive weight gain before it becomes too difficult to reverse.
To date, the majority of studies on child growth have focused on non-rural populations. Yet, rural youth are at greater risk for obesity and severe obesity (4, 39), and are more likely to display poor dietary patterns, sedentary behaviors, and live in poverty (40). This may explain, in part, why we did not observe differences in early sociodemographic risk by BMI trajectory that have been reported elsewhere (41). Perhaps, living in a poor, rural community may itself be a risk factor for obesity and severe obesity due to a number of unmeasured contextual factors. However, we did find that Black youth had increased odds of being in the “developed obesity” trajectory, compared with youth in the “maintained non-overweight” trajectory, which is consistent with past work (41). Yet, we also found that being Black did not predict membership in the “developed severe obesity” trajectory, suggesting that severe obesity may have its own unique pattern of obesity-related risk factors. These findings highlight the need to identify protective factors that reduce obesity risk in rural and racial minority populations.
Rapid growth in infancy has been associated with greater adiposity across the life span (42, 43). We extend these findings by demonstrating that infant birthweight (“developed severe obesity”), and weight-for-length as early as 2 months, and rapid weight gain in early infancy (“developed obesity”) predicted trajectory membership. Harrington et al suggest that the “tipping point” for some children with overweight and obesity occurs before age 3 months; obesity onset occurred before 2 years for the majority of youth in their study.(10) This is particularly problematic, given that steeper increases in BMI through adulthood have been noted in youth with severe obesity (17).
We found that breastfeeding was associated with a lower prevalence of both obesity and severe obesity, as it has been shown in past work (41, 44), and that early introduction of solids (≤2 months) was associated with the “developed severe obesity” trajectory. It is recommended (45) that infants be breastfed exclusively for at least 4 to 6 months, and that solid foods be introduced between 4 to 6 months. Only 14.7% of mothers in our study reported breastfeeding in the past week at the 6-month visit; 42.1% reported introducing solids by the 2-month visit. Low breastfeeding rates and early introduction of solids have been associated with lower socioeconomic status (46), and Gibbs et al suggest that infant feeding practices may partially explain the link between SES and obesity.(47) We add to this body of evidence by establishing a link between infant feeding and the development of severe obesity among rural, poor youth. These findings have implications for prevention, given the potentially low-cost and sustainability of interventions designed to alter infant feeding practices.
Higher maternal weight status measured at all visits predicted membership in the “developed obesity” and “developed severe obesity” trajectories, which is consistent with previous studies (30, 48). Additionally, more than twice the percentage of youth in the “developed severe obesity” trajectory and triple the percentage in the “developed obesity” trajectory had a mother with severe obesity, compared with youth in the “maintained non-overweight” trajectory. It is important to note that parents’ weight status represents both genetic and environmental contributions to youth obesity. Nearly 73.1% of youth in the “developed severe obesity” trajectory did not have a mother with severe obesity at the 24-month visit. It may be the case that factors contributing to the development of severe obesity in youth are context-based and largely malleable, and may be suitable targets for early intervention and prevention efforts.
Our study has several limitations. Our findings cannot be generalized to all rural youth in the U.S., although the sample is representative of rural, poor youth living in Pennsylvania and North Carolina. In addition, eating- and physical activity-related measures were not collected in this study, and it is possible that social desirability bias may have influenced maternal self-reports. Maternal height and weight at 2 and 6 months, and 12 years and infant birthweight were reported by mothers, which adds error due to recall bias. Further, maternal weight was reported 2 months after giving birth, which may partially reflect gestational weight gain. However, concerns are slightly diminished given that the findings for self-reported height and weight data were consistent with the measured height and weight data at 24 months. Lastly, the small size of the “developed severe obesity” trajectory limit our ability to compare across groups, and may have accounted for some marginal findings.
Our findings provide longitudinal evidence for early childhood risk factors associated with the development of obesity and severe obesity from early childhood through adolescence, including accelerated infant growth, shorter breastfeeding duration, early introduction of solids, and higher maternal weight status. Efforts to identify factors that confer early childhood protection against the development of obesity and severe obesity are needed (49). We also demonstrate the wide variability of BMI growth, and describe BMI trajectories among rural, poor children who develop obesity and severe obesity, highlighting the utility of methods that reveal, rather than compress, variability ≥95th BMI percentile. These graphing tools may prove useful in communicating obesity and severe obesity prevalence and trends to community, policy and decision-making stakeholders, and for identifying early life, sensitive periods for obesity prevention.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the FLP families and home visitors who contributed to this study.
This study utilizes data from the Family Life Project (FLP) [https://flp.fpg.unc.edu/]. Grant support for FLP data collection was provided by the National Institute of Child Health and Human Development (R01 HD081252 and P01 HD039667). The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR’S POTENTIAL CONFLICTS OF INTEREST: No competing financial interests exist.
REFERENCES
- 1.Bass R, Eneli I. Severe childhood obesity: an under-recognised and growing health problem. Postgrad Med J. 2015;91:639–45. [DOI] [PubMed] [Google Scholar]
- 2.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics. 2018;141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007–2008 to 2015–2016. Jama. 2018;319:1723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson JA 3rd, Johnson AM. Urban-rural differences in childhood and adolescent obesity in the United States: a systematic review and meta-analysis. Child Obes. 2015;11:233–41. [DOI] [PubMed] [Google Scholar]
- 5.Deckelbaum RJ, Williams CL. Childhood obesity: the health issue. Obes Res. 2001;9 Suppl 4:239S–43S. [DOI] [PubMed] [Google Scholar]
- 6.Daniels SR. The consequences of childhood overweight and obesity. Future Child. 2006;16:47–67. [DOI] [PubMed] [Google Scholar]
- 7.McTigue KM, Stepp SD, Moore CG, Cohen ED, Hipwell AE, Loeber R, et al. The development of youth-onset severe obesity in urban US girls. Journal of clinical & translational endocrinology. 2015;2:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munthali RJ, Kagura J, Lombard Z, Norris SA. Early Life Growth Predictors of Childhood Adiposity Trajectories and Future Risk for Obesity: Birth to Twenty Cohort. Child Obes. 2017;13:384–91. [DOI] [PubMed] [Google Scholar]
- 9.McGinty SM, Osganian SK, Feldman HA, Milliren CE, Field AE, Richmond TK. BMI Trajectories from Birth to Young Adulthood. Obesity. 2018;26:1043–9. [DOI] [PubMed] [Google Scholar]
- 10.Harrington JW, Nguyen VQ, Paulson JF, Garland R, Pasquinelli L, Lewis D. Identifying the “tipping point” age for overweight pediatric patients. Clin Pediatr (Phila). 2010;49:638–43. [DOI] [PubMed] [Google Scholar]
- 11.Mirza N, Phan TL, Tester J, Fals A, Fernandez C, Datto G, et al. Expert Exchange Workgroup on Children Aged 5 and Younger with Severe Obesity: A Narrative Review of Medical and Genetic Risk Factors. Child Obes. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bichteler A, Gershoff ET. Identification of Children’s BMI Trajectories and Prediction from Weight Gain in Infancy. Obesity. 2018;26:1050–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrissey TW. Trajectories of growth in body mass index across childhood: Associations with maternal and paternal employment. Social Science & Medicine. 2013;95:60–8. [DOI] [PubMed] [Google Scholar]
- 14.Freedman DS, Butte NF, Taveras EM, Lundeen EA, Blanck HM, Goodman AB, et al. BMI z-Scores are a poor indicator of adiposity among 2- to 19-year-olds with very high BMIs, NHANES 1999–2000 to 2013–2014. Obesity. 2017;25:739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. New Engl J Med. 2015;373:1307–17. [DOI] [PubMed] [Google Scholar]
- 16.Francis LA, Rollins BY, Bryce CI, Granger DA. Biobehavioral Dysregulation and its association with Obesity and Severe Obesity Trajectories from 2 to 15 years of age: A longitudinal study. Obesity. 2020;28:830–83. [DOI] [PubMed] [Google Scholar]
- 17.Freedman DS, Lawman HG, Galuska DA, Goodman AB, Berenson GS. Tracking and Variability in Childhood Levels of BMI: The Bogalusa Heart Study. Obesity. 2018;26:1197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedman DS, Butte NF, Taveras EM, Goodman AB, Blanck HM. Longitudinal changes in BMI z-scores among 45 414 2–4-year olds with severe obesity. Ann Hum Biol. 2017;44:687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile? Eur J Clin Nutr. 2005;59:419–25. [DOI] [PubMed] [Google Scholar]
- 20.Berkey CS, Colditz GA. Adiposity in adolescents: change in actual BMI works better than change in BMI z score for longitudinal studies. Ann Epidemiol. 2007;17:44–50. [DOI] [PubMed] [Google Scholar]
- 21.Organization WH. WHO child growth standards: head circumference-for-age, arm circumference-for-age, triceps skinfold-for-age and subscapular skinfold-for-age: methods and development. 2007. [Google Scholar]
- 22.Racette SB, Yu LY, DuPont NC, Clark BR. BMI-for-age graphs with severe obesity percentile curves: tools for plotting cross-sectional and longitudinal youth BMI data. BMC Pediatr. 2017;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smego A, Woo JG, Klein J, Suh C, Bansal D, Bliss S, et al. High Body Mass Index in Infancy May Predict Severe Obesity in Early Childhood. J Pediatr-Us. 2017;183:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ram N, Grimm KJ. Growth Mixture Modeling: A Method for Identifying Differences in Longitudinal Change Among Unobserved Groups. Int J Behav Dev. 2009;33:565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gittner LS, Ludington-Hoe SM, Haller HS. Infant Obesity and Severe Obesity Growth Patterns in the First Two Years of Life. Matern Child Hlth J. 2014;18:613–24. [DOI] [PubMed] [Google Scholar]
- 26.Flores G, Lin H. Factors predicting severe childhood obesity in kindergarteners. Int J Obesity. 2013;37:31–9. [DOI] [PubMed] [Google Scholar]
- 27.Andrea SB, Hooker ER, Messer LC, Tandy T, Boone-Heinonen J. Does the association between early life growth and later obesity differ by race/ethnicity or socioeconomic status? A systematic review. Ann Epidemiol. 2017;27:583–92.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horodynski MA, Pierce SJ, Reyes-Gastelum D, Olson B, Shattuck M. Feeding Practices and Infant Growth: Quantifying the Effects of Breastfeeding Termination and Complementary Food Introduction on BMI z-Score Growth Velocity through Growth Curve Models. Child Obes. 2017;13:490–8. [DOI] [PubMed] [Google Scholar]
- 29.Yan J, Liu L, Zhu Y, Huang G, Wang PP. The association between breastfeeding and childhood obesity: a meta-analysis. Bmc Public Health. 2014;14:1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitaker KL, Jarvis MJ, Beeken RJ, Boniface D, Wardle J. Comparing maternal and paternal intergenerational transmission of obesity risk in a large population-based sample. Am J Clin Nutr. 2010;91:1560–7. [DOI] [PubMed] [Google Scholar]
- 31.Vernon-Feagans L, Cox M. The Family Life Project: an epidemiological and developmental study of young children living in poor rural communities. Monographs of the Society for Research in Child Development. 2013;78:1–150, vii. [DOI] [PubMed] [Google Scholar]
- 32.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 33.Jung T, Wickrama K. An introduction to latent class growth analysis and growth mixture modeling. Social and personality psychology compass. 2008;2:302–17. [Google Scholar]
- 34.Kass RE, Raftery AE. Bayes factors. Journal of the american statistical association. 1995;90:773–95. [Google Scholar]
- 35.Schwarz G Estimating the dimension of a model. The annals of statistics. 1978;6:461–4. [Google Scholar]
- 36.Nylund KL, Asparouhov T, Muthén BO. Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo Simulation Study. Structural Equation Modeling: A Multidisciplinary Journal. 2007;14:535–69. [Google Scholar]
- 37.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. Bmj. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gulati AK, Kaplan DW, Daniels SR. Clinical Tracking of Severely Obese Children: A New Growth Chart. Pediatrics. 2012;130:1136–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogden CL, Fryar CD, Hales CM, Carroll MD, Aoki Y, Freedman DS. Differences in obesity prevalence by demographics and urbanization in us children and adolescents, 2013–2016. JAMA. 2018;319:2410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolin JN, Bellamy GR, Ferdinand AO, Vuong AM, Kash BA, Schulze A, et al. Rural Healthy People 2020: New Decade, Same Challenges. The Journal of Rural Health. 2015;31:326–33. [DOI] [PubMed] [Google Scholar]
- 41.Tester JM, Phan TLT, Tucker JM, Leung CW, Gillette MLD, Sweeney BR, et al. Characteristics of Children 2 to 5 Years of Age With Severe Obesity. Pediatrics. 2018;141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leunissen RJ, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA. 2009;301:2234–42. [DOI] [PubMed] [Google Scholar]
- 43.Shibli R, Rubin L, Akons H, Shaoul R. Morbidity of overweight (≥ 85th percentile) in the first 2 years of life. Pediatrics. 2008;122:267–72. [DOI] [PubMed] [Google Scholar]
- 44.Patro-Gołąb B, Zalewski BM, Kołodziej M, Kouwenhoven S, Poston L, Godfrey KM, et al. Nutritional interventions or exposures in infants and children aged up to 3 years and their effects on subsequent risk of overweight, obesity and body fat: a systematic review of systematic reviews. Obes Rev. 2016;17:1245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnston M, Landers S, Noble L, Szucs K, L. V Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–41. [DOI] [PubMed] [Google Scholar]
- 46.Gross RS, Mendelsohn AL, Fierman AH, Racine AD, Messito MJ. Food insecurity and obesogenic maternal infant feeding styles and practices in low-income families. Pediatrics. 2012;130:254–61. [DOI] [PubMed] [Google Scholar]
- 47.Gibbs BG, Forste R. Socioeconomic status, infant feeding practices and early childhood obesity. Pediatr Obes. 2014;9:135–46. [DOI] [PubMed] [Google Scholar]
- 48.Danielzik S, Langnäse K, Mast M, Spethmann C, Müller MJ. Impact of parental BMI on the manifestation of overweight 5–7 year old children. Eur J Nutr. 2002;41:132–8. [DOI] [PubMed] [Google Scholar]
- 49.Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe Obesity in Children and Adolescents: Identification, Associated Health Risks, and Treatment Approaches A Scientific Statement From the American Heart Association. Circulation. 2013;128:1689–712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


