Figure 2.
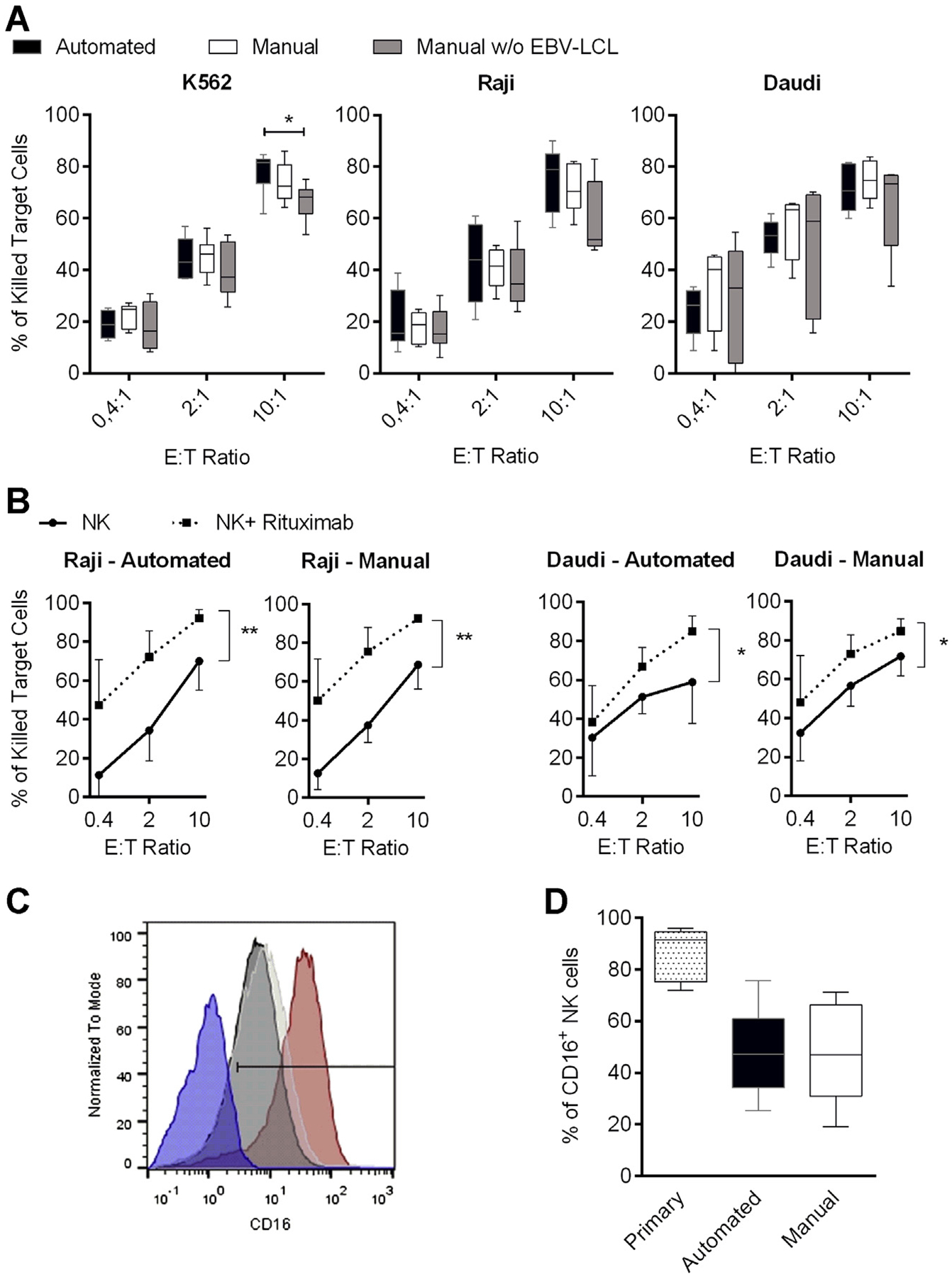
Natural and antibody-dependent cytotoxicity of differentially expanded NK cells. (A) NK cells were expanded for 14 days by means of the automated process (black bars) in comparison to manual NK cell expansion in with (white bars) or without (gray bars) EBV-LCL and analyzed for cytotoxicity against K562, Raji and Daudi cell lines at different effector-to-target (E:T) ratios. (B) Cytotoxicity against CD20-positive Raji or Daudi cells that were untreated (circles) or treated (squares) with 1 μg/mL rituximab for 4 h during the assay. P values indicated are for paired Student’s t-test, with P < 0.05 considered significant. Six or seven donors were analyzed; displayed are mean values, minimum to maximum (whiskers in A) and standard deviation. (C) Representative CD16 expression on NK cells from one donor is shown. Comparison of primary NK cells (red curve) and NK cells after 14 days of automated (black curve) or manual (white curve) expansion in the presence of EBV-LCL is displayed. CD16-negative PBMCs served as a control to determine the positive gate (blue curve). (D) Overview of the CD16 staining of all seven donors used in B is depicted; displayed are mean values, minimum to maximum and standard deviation.
