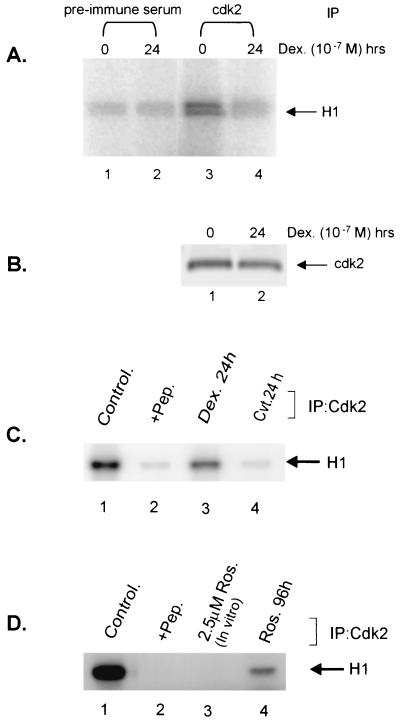
FIG. 1.
Glucocorticoids and CVT-313 block phosphorylation of histone H1 in Vitro. (A) Prolonged glucocorticoid treatment inhibits cdk2 activity. Mouse mammary cells were untreated (lanes 1 and 3) or treated with dexamethasone (10−7 M) for 24 h (lanes 2 and 4). cdk2 (lanes 3 and 4) was immunoprecipitated from 100 μg of total proteins in whole-cell lysate. As a control, an identical reaction was carried out with a preimmune serum (lanes 1 and 2). Immunocomplexes were then incubated with purified histone H1 in presence of [γ-32P]ATP for 10 min at 25°C. Samples were analyzed by SDS-PAGE, followed by autoradiography. (B) The Western blot below the kinase gel indicates the level of cdk2 kinase immunoprecipitated by the anti-CDK2 antibody from the same cellular lysates used for the kinase reaction in panel A. (C) CVT-313 inhibits H1 phosphorylation in vitro. Cells were untreated (lanes 1 and 2) or treated with dexamethasone (lane 3) or CVT-313 25 μM (lane 4) for 24 h. cdk2 was immunoprecipitated from cell lysate with affinity-purified anti-cdk2 antibodies (lanes 1, 3, and 4) or anti-cdk2 antibodies preabsorbed with an excess of peptide antigen (lane 2). The kinase reactions were performed as described above. (D) Roscovitine inhibits H1 phosphorylation in vitro. Cells were untreated (lanes 1, 2, and 3) or treated with Roscovitine at 25 μM (lane 4) for 96 h. cdk2 was immunopurified with purified anti-cdk2 antibodies (lanes 1, 3, and 4) or anti-cdk2 antibodies preabsorbed with an excess of peptide antigen (lane 2). Immunopurified cdk2 was then incubated in vitro with 2.5 μM Roscovitine (lane 3) prior to the kinase reaction. The samples were analyzed as in panel A.