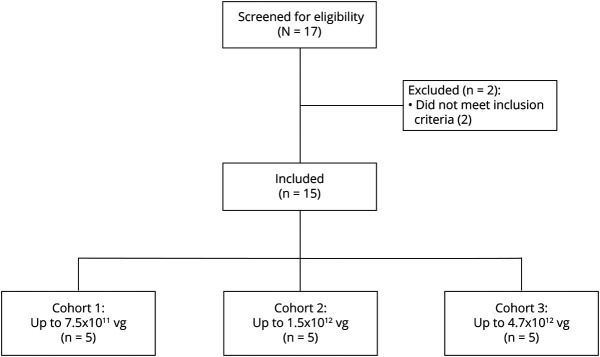
Figure 1. Participant Flow Diagram for the PD-1101 Trial.
PD-1101 was a phase 1b, open-label, dose escalation trial of VY-AADC01 in participants with moderately advanced Parkinson disease. Of the 2 participants excluded from enrollment, 1 participant had an elevated anti-AAV2 antibody titer and the other participant had a modified Hoehn & Yahr score below the inclusion criteria. Participants were screened for eligibility within 60 days of VY-AADC01 administration and followed for 36 months after administration. All participants were included in analyses.