Figure 4.
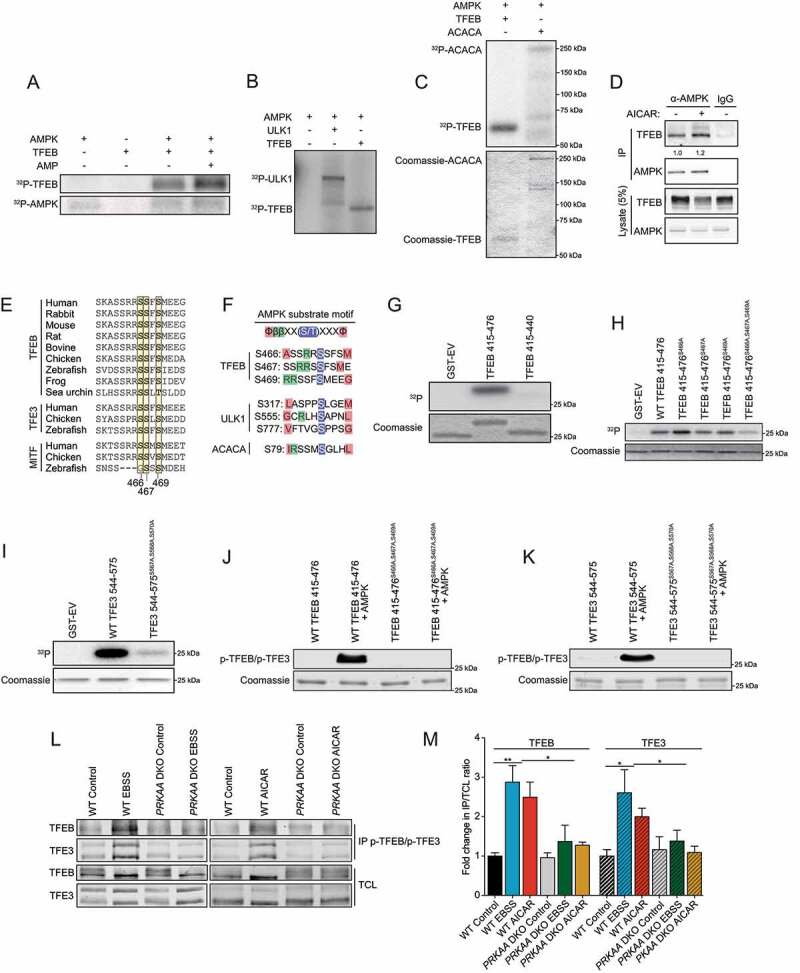
AMPK phosphorylates TFEB and TFE3 on highly conserved serine cluster 466, 467, 469. (A) In vitro kinase assays. Purified AMPK (0.1 μg) and recombinant TFEB (0.5 μg) were incubated with [γ-32P]ATP with or without AMP and analyzed by SDS-PAGE followed by autoradiography. Data are representative of three independent experiments. (B) In vitro kinase assays. Purified AMPK (0.1 μg), recombinant TFEB (0.5 μg), and recombinant ULK1 (0.5 μg) incubated as described in (A) (C) In vitro kinase assays. Purified AMPK (0.05 μg), recombinant TFEB (0.5 μg), and recombinant ACACA (0.5 μg) incubated as described in (A). (D) Endogenous protein complexes were immuno-purified from HEK293T cells treated with vehicle or 2 mM AICAR for 2 h with AMPKα1/2 antibody and analyzed by immunoblotting with indicated antibodies. Nonspecific IgG was used as a negative control. Data are representative of three independent experiments, quantification of TFEB protein levels normalized to AMPK protein level, Student’s t-test, p = 0.03. (E) Alignment of TFEB, TFE3, and MITF amino acid C-terminal regions from various species. (F) AMPK substrate motif alignment surrounding the serine residues 466, 467 and 469 in human TFEB, ULK1, and ACACA. (G) In vitro kinase assays using [γ-32P]ATP, Purified AMPK, and GST-TFEB fragments purified from bacteria as described and analyzed by SDS-PAGE followed by Coomassie staining and autoradiography. Data are representative of three independent experiments. (H) In vitro kinase assays using [γ-32P]ATP, Purified AMPK, and GST-TFEB fragments purified from bacteria as described and analyzed by SDS-PAGE followed by Coomassie staining and autoradiography. Data are representative of three independent experiments. (I) In vitro kinase assays using [γ-32P]ATP, Purified AMPK, and GST-TFE3 fragments purified from bacteria as described and analyzed by SDS-PAGE followed by Coomassie staining and autoradiography. Data are representative of three independent experiments. (J-K) In vitro kinase assay using cold ATP, Purified AMPK, and GST-TFEB or GST-TFE3 fragments purified from bacteria and analyzed by immunoblotting with the phospho-specific TFEB and TFE3 antibody. (L) Endogenous phosphorylated TFEB and TFE3 were immunoprecipitated with pTFEB/pTFE3 antibody followed by immunoblotting using either total TFEB or total TFE3 antibodies. WT HEK293T or prkaa DKO cells were incubated in complete media (Control), starved (EBSS), or in presence of AICAR (2 mM) for 2 h. Data are representative of three independent experiments. (M) Quantification of the fold change in pTFEB and pTFE3 purified compared to TFEB and TFE3 in total cell lysates (TCL) ratio upon treatment as described in (L) (mean ± SEM of three independent experiments, two-way anova, *P < 0.05; **P < 0.01)
