Figure 4.
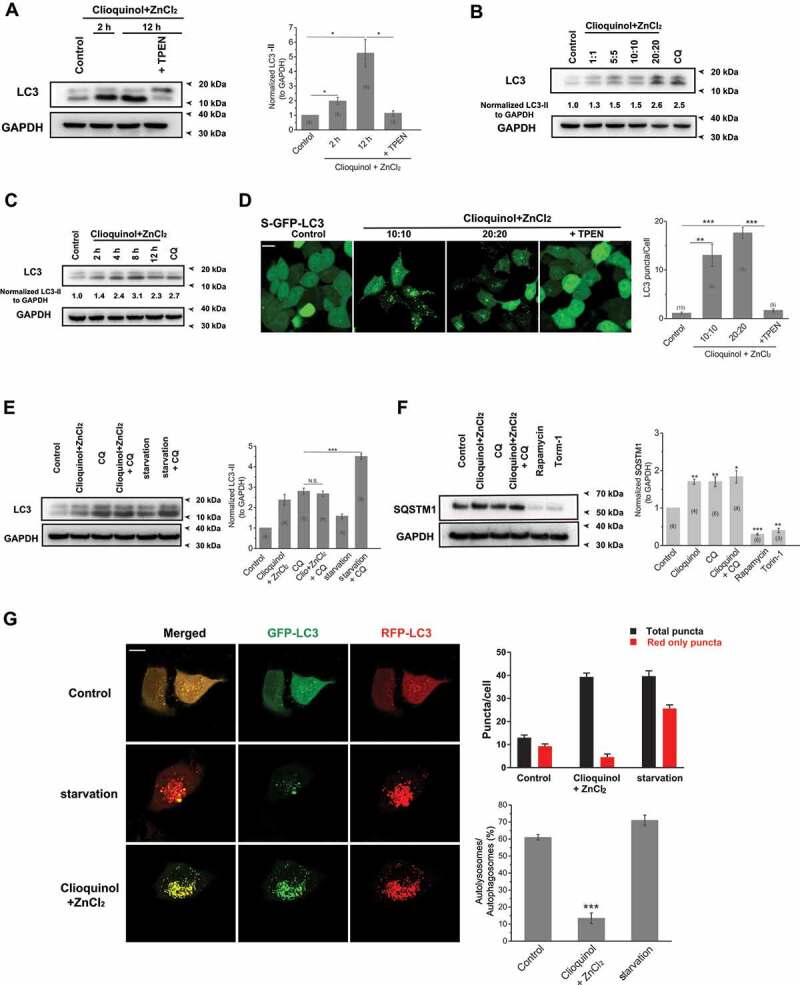
Zinc influx originating from the extracellular fluid arrests autophagy. (A) Zinc influx induced by co-application of ZnCl2 (20 μM) and clioquinol (20 μM) significantly increased LC3-II levels in HeLa cells, confirmed by the western blot experiments. TPEN (10 μM) profoundly reduced increased LC3-II levels induced by ZnCl2 and clioquinol. (B, C) The increases in LC3-II levels caused by co-application of ZnCl2 and clioquinol in HeLa cells were dose- (B; 4 h treatments) and time-dependent (C; 20 μM clioquinol + 20 μM ZnCl2 used here). (D) Co-application of clioquinol and ZnCl2 induced LC3 puncta in GFP-LC3 stably expressing HEK 293 T (S-GFP-LC3) cells and LC3 puncta structures were reduced by co-application of TPEN (10 μM). All treatments were for 4 h. Scale bar: 10 μm. (E) CQ (10 μM) did not further increase LC3-II levels than clioquinol alone (20 μM clioquinol + 20 μM ZnCl2) in HeLa cells, whereas it facilitated LC3-II levels increased by starvation (AA- and FBS-free). (F) SQSTM1 levels were measured under different treatments in HeLa cells including the control, 20 μM clioquinol + 20 μM ZnCl2, CQ (10 μM), CQ+ clioquinol + ZnCl2, rapamycin (50 μM), and torin-1 (1 μM). Rapamycin and torin-1 served as positive controls for inducing autophagy (reducing SQSTM1 levels). (G) In GFP-RFP-LC3 transiently expressed HeLa cells, application of ZnCl2 and clioquinol (20 μM+20 μM) caused both increased green and red puncta in the same pace, while starvation (AA- and FBS-free) induced much more red puncta over green puncta. All treatments were for 4 h. Scale bar: 10 μm. The numbers of total (autophagosomes) and red only puncta (autolysosomes) and ratio of autolysosomes to autophagosomes were quantified from three independent experiments (typically n = 6–13 cells). Error bars indicate Mean ± SEMs in panels A, D, E, F and G. Significant differences were evaluated using one-way ANOVA followed by Tukey’s test. *P < 0.05; **P < 0.01; ***P < 0.001
