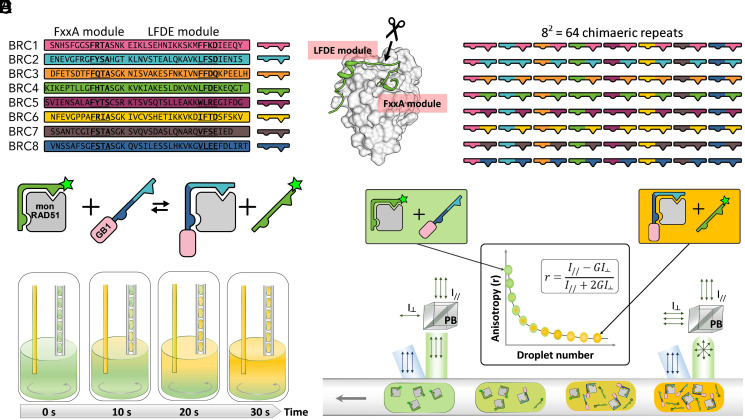
Fig. 1.
Shuffling of the binding modules comprising the eight RAD51-binding repeats in BRCA2. (A) The eight human BRC repeats with FxxA and LFDE motifs in bold. (B) The crystal structure of RAD51:BRC4 peptide complex (PDB ID: 1n0w). The arrow with scissors indicates the crossover point between the FxxA and LFDE modules used in this study. (C) Schematic representation of the 56 chimeric and eight natural repeats resulting from the shuffling around the crossover point described in B. (D) Schematic representation of the competition assay used for the affinity determination of the BRC-repeat shuffle set for monomeric RAD51 (mon RAD51) by fluorescence anisotropy. GB1-BRC recombinant peptide fusions were titrated into a complex of monomeric RAD51 and BRC4fl (a fluorescein-labeled BRC4 synthetic peptide), so that the Kd of the recombinant peptide could be calculated using the known Kd of BRC4fl 49,50. (E) Titration of GB1-BRC peptides using droplets on demand: microdroplets containing an increasing concentration of fusion peptides were produced from a well of a 384-well plate by a microcapillary aspiration technique. During 30 s, a stock solution of GB1-BRC peptide was injected in the well so that its concentration rose from 0 to 50% of stock concentration (left capillary). Simultaneously, droplets encapsulating the changing contents of the well were generated (right capillary). Flow directions are represented by gray arrows. Mixing was performed by a magnetic stir bar (gray circling arrow). The concentration of monomeric RAD51 and BRC4fl was kept constant throughout the titration. (F) The titration droplets were measured for their average fluorescence anisotropy using a fluorescence anisotropy imaging microscope. Upon traveling above the imaging area, linearly polarized light (in blue) excited the fluorescein tags of the BRC4fl peptides, either bound to monomeric RAD51 (larger hydrodynamic volume, resulting in high anisotropy) or freely tumbling after competition with GB1-BRC peptides (lower hydrodynamic volume, resulting in low anisotropy). Fluorescence emission intensity signals (in green) recorded for parallel and perpendicular polarizations enabled the quantification of average anisotropy for every droplet of the titration sequence. A G factor was calculated to correct for differences in detector sensitivities in the parallel and perpendicular channels. PB, polarizing beamsplitter.