Abstract
BACKGROUND
Three-dimensional (3D) modelling technology translates the patient-specific anatomical information derived from two-dimensional radiological images into virtual or physical 3D models, which more closely resemble the complex environment encountered during surgery. It has been successfully applied to surgical planning and navigation, as well as surgical training and patient education in several surgical specialties, but its uptake lags behind in colorectal surgery. Rectal cancer surgery poses specific challenges due to the complex anatomy of the pelvis, which is difficult to comprehend and visualise.
AIM
To review the current and emerging applications of the 3D models, both virtual and physical, in rectal cancer surgery.
METHODS
Medline/PubMed, Embase and Scopus databases were searched using the keywords “rectal surgery”, “colorectal surgery”, “three-dimensional”, “3D”, “modelling”, “3D printing”, “surgical planning”, “surgical navigation”, “surgical education”, “patient education” to identify the eligible full-text studies published in English between 2001 and 2020. Reference list from each article was manually reviewed to identify additional relevant papers. The conference abstracts, animal and cadaveric studies and studies describing 3D pelvimetry or radiotherapy planning were excluded. Data were extracted from the retrieved manuscripts and summarised in a descriptive way. The manuscript was prepared and revised in accordance with PRISMA 2009 checklist.
RESULTS
Sixteen studies, including 9 feasibility studies, were included in the systematic review. The studies were classified into four categories: feasibility of the use of 3D modelling technology in rectal cancer surgery, preoperative planning and intraoperative navigation, surgical education and surgical device design. Thirteen studies used virtual models, one 3D printed model and 2 both types of models. The construction of virtual and physical models depicting the normal pelvic anatomy and rectal cancer, was shown to be feasible. Within the clinical context, 3D models were used to identify vascular anomalies, for surgical planning and navigation in lateral pelvic wall lymph node dissection and in management of recurrent rectal cancer. Both physical and virtual 3D models were found to be valuable in surgical education, with a preference for 3D printed models. The main limitations of the current technology identified in the studies were related to the restrictions of the segmentation process and the lack of 3D printing materials that could mimic the soft and deformable tissues.
CONCLUSION
3D modelling technology has potential to be utilised in multiple aspects of rectal cancer surgery, however, it is still at the experimental stage of application in this setting.
Keywords: Rectal cancer, Three-dimensional modelling, Three-dimensional printing, Image-guided surgery, Surgical navigation, Surgical education
Core Tip: Three-dimensional (3D) modelling technology has revolutionized preoperative planning, intraoperative navigation, and surgical training in several surgical specialties. Rectal cancer surgery poses significant challenges due to the complex anatomy of the pelvis. While there is marked interest in the application of 3D modelling in this field, it appears to be still in its relative infancy. Future research and technological developments will enable clinical application of the virtual and physical 3D models to enhance surgical vision before and during rectal cancer surgery.
INTRODUCTION
Colorectal cancer is the second leading cause of cancer deaths worldwide[1]. Cancer of the rectum accounts for approximately 30% of all colorectal malignancies. Rectal cancer surgery has undergone revolutionary changes within the last three decades. The standard application of the total mesorectal excision (TME), the use of combination of chemo- and radiotherapy and the advent of minimally invasive approaches have all contributed to the improvement of patients’ surgical and oncological outcomes[2,3]. However, rectal cancer surgery still poses significant technical challenges due to the complex anatomy of the pelvis, which contains crucial digestive, urinary and gynaecological organs, surrounded by the intimately interlinked minute pelvic nerves and vessels, all together enclosed within a rigid and often narrow space.
Obtaining the correct diagnosis and formulating a comprehensive management plan requires an effective multidisciplinary communication between the radiologists, surgeons and oncologists, which heavily relies on the radiological investigations. Magnetic resonance imaging (MRI) has become the gold standard in rectal cancer assessment[4]; however, it can be very difficult to comprehend for a non-expert eye. The use of three-dimensional (3D) models, both virtual and 3D printed, presents the information obtained from the two-dimensional radiological images in a way that resembles the complex 3D pelvic space encountered intraoperatively.
3D models have been found beneficial to all aspects of surgical care, from the recognition of patient’s individual anatomy and creation of precise surgical roadmap, through surgical education to patient interaction[5,6].
Within the colorectal surgery, 3D imaging is used in computed tomography (CT) colonography where it provides the “fly-through” views of the colon, and in 3D reconstruction of CT angiography, which has already become a routine part of preoperative planning for cancer segmental colectomies in many institutions[7,8]. While the use of these two modalities has been thoroughly reported, the use of 3D modelling technology in rectal cancer surgery has not been reviewed. The two most recent systematic reviews of the applications of 3D printing in colorectal surgery identified only one paper addressing its use in rectal surgery, however these systematic reviews did not address the use of 3D virtual models[9,10].
This systematic review aims to provide a comprehensive picture of the current role of the 3D modelling technology in rectal cancer surgery and to identify the future directions of exploration of its application.
MATERIALS AND METHODS
Literature search
Electronic databases, PubMed/MEDLINE, Embase and Scopus, were searched to identify studies describing the use of 3D models, both virtual and physical, in rectal cancer surgery between 2000 and 2020. Keywords in the search strategy included: “3D”, ”three-dimensional”, “model”, “colorectal”, “rectum”, “surgery”, “planning”’, “navigation”, “simulation”, “surgical education”, “patient education”. The reference section of each paper was further screened for other relevant papers.
Inclusion criteria
All full-text studies published in English, which described 3D virtual or physical models used in any aspect of rectal cancer surgery were considered eligible for inclusion, regardless of study type.
Exclusion criteria
Duplicate articles, review papers and conference abstracts were excluded. Studies in which 3D models were derived from animals or cadavers, as well as studies of pelvic volumetry and radiotherapy planning were excluded.
Screening and data extraction
Title and abstract screening were performed independently by two reviewers (Przedlacka A and Fletcher J). The cases where consensus was not achieved, were resolved by Kontovounisios C and Pellino G. Full-text review and data extraction were performed independently by two reviewers (Przedlacka A and Kontovounisios C). The manuscript was drafted by Przedlacka A and revised by all authors.
The following information was extracted from each study: Author, year of publication, country where study was conducted, patient demographics, indication for 3D modelling, type of model (virtual or physical), methodology of image segmentation, time and cost of 3D modelling and 3D printing, study outcomes and limitations. The manuscript was prepared and revised according to the PRISMA 2009 Checklist[11].
RESULTS
Study characteristics
The details of the study screening are presented in Figure 1. Sixteen studies were found to be eligible for inclusion in the present systematic review. There were 8 studies from Asia, 7 from Europe and one from the United States. The studies were published between 2006 and 2020, with 14 out of 16 published since 2017. There was one single-centre open-label randomised controlled trial, 4 retrospective studies, 9 feasibility or pilot studies and 2 case reports. The characteristics of the studies and their participants are shown in Table 1. The application of 3D modelling in each study is presented in Table 2.
Figure 1.
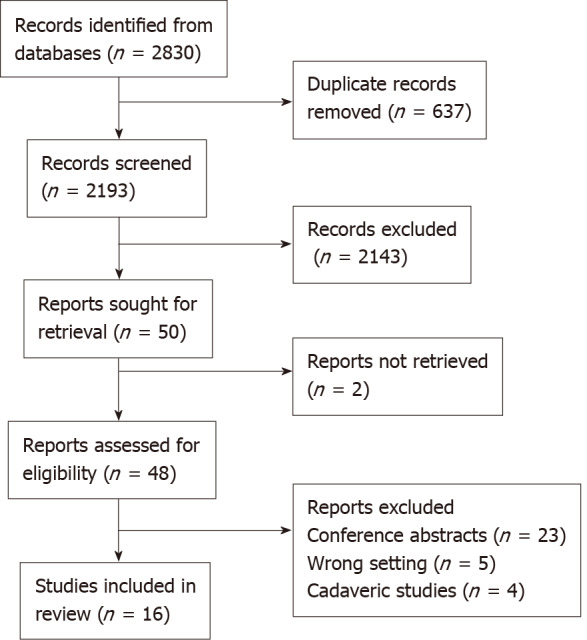
PRISMA flowchart.
Table 1.
Characteristics of the studies and participants
|
Ref.
|
Country
|
Study type
|
Number of participants
|
Age (yr)
|
Gender (male/female)
|
| Kontovounisios et al[10], 2019 | United Kingdom | Feasibility | 10 | No data | 5/5 |
| Hamabe et al[11], 2017 | Japan | Feasibility | 2 | No data | 1/1 |
| Sahnan et al[12], 2018 | United Kingdom | Feasibility | 2 | No data | 2/0 |
| Przedlacka et al[13], 2020 | United Kingdom | Feasibility | 30 | No data | No data |
| Garcia-Granero et al[14], 2020 | Spain/Italy | Feasibility | 2 | No data | 2/0 |
| Garcia-Granero et al[15], 2020 | Spain/Italy | Feasibility | 2 | No data | 1/1 |
| Sueda et al[16], 2019 | Japan | Case report | 1 | 83 | 0/1 |
| Chen et al[17], 2020 | China | Case report | 1 | 68 | 1/0 |
| Kim et al[18], 2020 | South Korea | Prospective observational | 10 | Median 60; range (40-80) | 8/2 |
| Hojo et al[19], 2020 | Japan | Retrospective Qualitative | 30 | No data | No data |
| Horie et al[20], 2018 | Japan | Retrospective | 10 | Median 62; range (43-77) | 8/2 |
| Hojo et al[21], 2020 | Japan | Retrospective | 11Rectal cancer: 5 | Median 67; range (56-79) | 6/5 |
| Nijkamp et al[22], 2018 | The Netherlands | Feasibility | 33Rectal cancer: 8 | No data | No data |
| Hassinger et al[23], 2020 | United States | Pilot study | 10 | No data | No data |
| Hojo et al[24], 2019 | Japan | Single-centre randomised controlled | 102 | No data | |
| Brannigan et al[25], 2006 | Belgium | Feasibility | 6 | Mean 66.5; range (54-81) | 3/3 |
Table 2.
Application of the three-dimensional modelling technology
|
Ref.
|
Pathology
|
Surgical procedure
|
Application
|
Main findings
|
| Kontovounisios et al[10] | Normal pelvis | NA | NA | Feasibility of construction of virtual 3D models of pelvis |
| Hamabe et al[11] | Normal pelvisRectal cancer | NA | NA | Feasibility of construction of 3D printed models of pelvis and rectal cancer |
| Sahnan et al[12] | Low rectal cancerUlcerative colitis | TaTME | NA | Feasibility of application of 3D models in surgical planning of TaTME |
| Przedlacka et al[13] | Rectal cancer T1-T4 | NA | Preoperative planning | Feasibility of construction of virtual 3D models of T stages of rectal cancer |
| Garcia-Granero et al[14] | Locally advanced rectal cancer | TME with en block prostatectomyTotal pelvic exenteration | Preoperative planning | Feasibility of application of a mathematical method to generate 3D models and assess prostate invasion in men with rectal cancer |
| Garcia-Granero et al[15] | Locally advanced primary and recurrent rectal cancer | Beyond TME | Preoperative planning | Feasibility of application of a mathematical method to generate 3D models and assess CRM status |
| Sueda et al[16] | Upper rectal cancer | Laparoscopic anterior resection | Preoperative planning | Identification of Retzius venous short circuit prior to laparoscopic anterior resection |
| Chen et al[17] | Rectal cancer (T3N2Mx) | Laparoscopic-assisted radical resection of rectum | Preoperative planning | Preoperative recognition of situs inversus |
| Kim et al[18] | Rectal cancer with metastatic LPNs | TME with LPLND | Preoperative planning and navigation | Index LPNs among ICG-bearing lymph nodes can be identified intraoperatively by matching 3D models |
| Hojo et al[19] | Rectal cancer with metastatic LPNs | LPLND | Preoperative planning and navigation | 3D -printed models are useful for surgical planning of LPLND, especially in cases with LPN metastases |
| Horie et al[20] | Advanced low rectal cancer | TME, tumour-specific mesorectal resection or total proctocolectomy with LPLND | Preoperative planning | 3D reconstruction revealed vascular anatomy variations in 40% |
| Hojo et al[21] | Infra-renal recurrence of colorectal cancer | Curative resection beyond TME | Preoperative planning and navigation | Usefulness of 3D models in surgical planning and navigation for resection of infra-renal recurrence of colorectal cancer, including rectal cancer |
| Nijkamp et al[22] | Locally advanced primary and recurrent rectal cancer | Resection of tumour | Intraoperative navigation | Feasibility of integration of 3D model into the novel EM- based navigation system |
| Hassinger et al[23] | Normal pelvic anatomy | NA | Surgical education | VAPS teaches clinically relevant anatomy and is preferred to traditional methods. More detailed model is required |
| Hojo et al[24] | Lower rectal cancer | Relevant to LPLND | Surgical education | 3D virtual and printed models are useful for teaching LPLND |
| Brannigan et al[25] | Middle and lower rectal cancer | Laparoscopic resection of rectal cancer | Surgical device design | The optimal angulation of a stapling device for transverse rectal transection is between 62º and 68º |
TaTME: Transanal total mesorectal excision; TME: Total mesorectal excision; CRM: Circumferential resection margin; LPN: Lateral pelvic sidewall lymph nodes; LPLND: Lateral pelvic lymph node dissection; VAPS: Virtual pelvic anatomy simulator.
The use of virtual 3D models was reported in 13 studies, 3D printed models in one and both types of models in two studies. Models were derived from CT scans in 8 studies, from MRI scans in five studies, while the combination of both modalities was used in two studies. Further characteristics of the methodology of 3D modelling and 3D printing described in studies are presented in Table 3.
Table 3.
Details of the three-dimensional model creation process
|
Study
|
3D model
|
Radiological modality
|
Segmentation
|
Segmentation performed by
|
Segmentation time
|
3D Printing time
|
3D printing material
|
| Kontovounisios et al[10] | Virtual | MRI | Manual | No data | No data | NA | NA |
| Hamabe et al[11] | Printed | CT | Manual | Colorectal Surgeon and Technician | 40 h | M – 37 h 30 min; F – 34 h 20 min | Ultraviolet-curated resin |
| Sahnan et al[12] | Virtual | MRI | Manual | Consultant gastrointestinal radiologist | Segmentation: 15 minSmoothing: 10 min | NA | NA |
| Przedlacka et al[13] | Virtual | MRI | Manual | No data | No data | NA | NA |
| Garcia-Granero et al[14] | Virtual | MRI | 3D-IPR | No data | No data | NA | NA |
| Garcia-Granero et al[15] | Virtual | MRI | 3D-IPR | No data | No data | NA | NA |
| Sueda et al[16] | Virtual | CT | No data | No data | No data | NA | NA |
| Chen et al[17] | Virtual | CT/MRI | No data | No data | No data | NA | NA |
| Kim et al[18] | Virtual | CT | No data | No data | No data | NA | NA |
| Hojo et al[19] | Virtual/printed | CT | Manual | Colorectal surgeon | No data | 40 h (decreased with experience) | No data |
| Horie et al[20] | Virtual | CT | No data | No data | No data | NA | NA |
| Hojo et al[21] | Virtual | No data | No data | No data | No data | No data | NA |
| Nijkamp et al[22] | Virtual | CT | Automatic (bones); Semi-automatic (arteries); Manual (other structures) | No data | 1-3 h | NA | |
| Hassinger et al[23] | Virtual | CT/MRI | No data | No data | No data | NA | |
| Hojo et al[24] | Virtual/Printed | CT | No data | Colorectal Surgeon and Radiologist | No data | 22 h | |
| Brannigan et al[25] | Virtual | CT | Semi-automatic | No data | No data | NA | NA |
MRI: Magnetic resonance imaging; CT: Computed tomography; 3D-IPR: Three-dimensional image processing and reconstruction.
For the purpose of the descriptive presentation of the results of the present systematic review, the studies were divided into four categories: (1) Feasibility of application of 3D modelling technology in rectal cancer surgery; (2) Surgical planning and navigation; (3) Surgical education; and (4) Surgical device design.
Feasibility of application of 3D modelling technology in rectal cancer surgery
Feasibility of construction of 3D models of normal pelvic anatomy: Kontovounisios et al[12] constructed 10 models of healthy volunteers (5 males and 5 females) to demonstrate the feasibility of creation of virtual models of normal pelvic anatomy. MRI images were manually segmented in ITK-SNAP and further post-processing was applied in MeshLab. The particular focus was placed on the central pelvic compartment, which contains the rectum, intra/extra-luminal fat and the mesorectum, and is relevant to the TME resection. The authors noted that the methodology could be applied to create models of rectal cancer, which could be utilised for surgical planning and patient consultation.
Hamabe and Ito[13] explored the feasibility of creation of a 3D printed model of pelvic anatomy relevant to rectal cancer surgery and specifically to lateral pelvic lymph node (LPN) dissection. The CT images of a healthy male volunteer and a female with rectal cancer were manually segmented to create 3D replicas of patients’ anatomy, including pelvic bones, pelvic floor muscles, internal and external iliac vessels with their branches, nerves and urogenital organs. The central compartment with the mesorectum and the rectum were not included in the models. The full-sized models were 3D printed with ultraviolet-cured resin. They could be cleaved in a sagittal plane to allow for the inspection of the deep parts.
Feasibility of construction of 3D models of rectal cancer: Sahnan et al[14] presented the feasibility of construction of two 3D virtual models for surgical planning of transanal TME (TaTME). These were created through manual segmentation of standard axial T2-weighted Spectral Attenuated Inversion Recovery sequences performed by a specialist consultant gastrointestinal radiologist. In the first case of a male patient with low rectal cancer, the model provided insight into the location at which the tumour penetrated the rectal wall and demonstrated the close relation but clearance of the tumour from the prostate and the urinary system. In the second case of a male with ulcerative colitis who was scheduled for combined single incision laparoscopy and TaTME completion proctectomy and ileoanal pouch, it provided an understanding of the anatomical landmarks and the insight into the relation between the internal sphincter and rectum, as well as between the prostate and urethra.
Przedlacka et al[15] reported constructing thirty 3D virtual models derived from the MRI T2 weighted sequences of patients with rectal cancer. The authors showed the feasibility of manual segmentation of the rectal wall layers to present the difference in the 3D appearance of T1 and T3 tumours. The authors also presented a model demonstrating infiltration of the prostatic gland in a T4 tumour. The models of early rectal cancer which comprise the central compartment only can be utilised for the assessment of suitability for the local excision of rectal cancer, while models of advanced tumours which display the central compartment in the context of the entire pelvic anatomy can be applied for preoperative planning of the beyond-TME surgery.
Garcia-Granero et al[16] presented the feasibility of application of a mathematical 3D-based model of image processing and reconstruction (3D-IPR) method to generate virtual 3D models of pelvis and to assess the invasion of the prostate by the rectal cancer. Two cases demonstrate the use and the diagnostic reliability of 3D-IPR models based on preoperative pelvic MRI and correlated with pathology as reference standard. A 60-year-old male with locally advanced primary rectal cancer was found to have infiltration of levator ani muscle and prostate with an uncertain urethral invasion on the MRI scan. Contrary to that, the 3D-IPR model showed infiltration of the puborectalis muscle, but neither prostate nor urethra was invaded. Patient underwent abdominoperineal excision with TME and partial en bloc prostatectomy with neoadjuvant chemoradiotherapy. Pathology showed R0 resection with no residual tumour cells in the prostate gland.
The second case illustrates a patient with ulcerative colitis and locally advanced primary rectal cancer infiltrating the puborectalis muscle and the prostate, treated with neoadjuvant chemoradiotherapy. The post-treatment MRI showed low tumour regression with persistent infiltration of the puborectalis muscle and the prostate gland. The 3D-IPR reconstruction based of the post-treatment MRI showed infiltration of the puborectalis muscle bilaterally and the prostate. Patient underwent total pelvic exenteration. The histopathology report confirmed a mucinous adenocarcinoma infiltrating the puborectalis muscle and the prostate with R0 resection.
In a separate study[17], the feasibility and diagnostic reliability of the same mathematical approach with 3D-IPR model based on pelvic MRI was evaluated in the assessment of the circumferential resection margin in two patients with locally advanced primary and recurrent rectal cancer. In the first case, the MRI reported locally advanced rectal cancer infiltrating the posterior vaginal wall and the internal sphincter with dubious external sphincter infiltration. 3D-IPR confirmed infiltration of these structures but indicated clearance of the external sphincter. Patient underwent neoadjuvant chemoradiotherapy followed by inter-sphincteric anterior resection of the rectum extended into posterior vaginal wall. Pathology showed presence of fibrosis and acellular mucin pools in the posterior vaginal wall and internal sphincter and confirmed that the R0 resection was achieved. In the second case of a patient who had previously undergone anterior resection for rectal cancer, MRI images showed pelvic sidewall recurrence infiltrating the levator ani muscle and the left obturator muscle without bone infiltration. 3D-IPR also indicated the invasion of the levator ani and the left obturator muscles but additionally, it suggested the infiltration of the left seminal vesicle and the left ischial spine. Patient underwent abdominoperineal excision extending to the pelvic periosteal lamina. Pathology showed R1 resection with the invasion of the left seminal vesicle, levator ani, obturator muscle and positive CRM at the bone surface as indicated by the 3D-IPR[14].
Application of 3D modelling technology in preoperative planning and intraoperative navigation in rectal cancer surgery
Preoperative recognition of vascular anatomy: Sueda et al[18] reported the usefulness of the 3D reconstruction of the CT images in pre-operative planning in an 83-year-old Japanese woman with upper rectal cancer and an unexpected finding of a rare venous malformation - the Retzius venous short circuit between the inferior mesenteric vein and the inferior vena cava. During laparoscopic anterior resection, the Retzius vein and the inferior mesenteric vein were ligated without bleeding, and the mesorectal excision was successfully completed.
Chen et al[19] described the application of preoperative recognition of anatomy which enhanced surgical planning in a 68-year-old Chinese woman with rectal cancer (T3N2Mx) and situs inversus. Preoperative identification of the congenital anomaly through the use of 3D virtual reconstruction of patient’s radiological images (CT and MRI) with Mimics system (Materialise) allowed for the safe completion of laparoscopic-assisted radical resection of rectal cancer with distal ileostomy.
LPN dissection: Kim et al[20] described the use of 3D reconstruction of preoperative CT images for surgical planning and intraoperative navigation during LPN dissection (LPLND). Thirteen patients scheduled to undergo TME with LPLND for rectal cancer were prospectively enrolled in the study. 3D images were constructed through volume rendering and depicted bones and essential structures in the pelvic sidewall, such as the obturator nerve and muscles, arteries and index LPNs, defined as metastatic LPNs identified on pre-treatment MRI. During surgery, LPNs were removed under the guidance of real-time fluorescence imaging with indocyanine green (ICG). The surgeon verified the position of the index nodes with 3D reconstruction images displayed on the computer or the console monitor in the case of robotic surgery. All index LPNs among ICG-bearing lymph nodes were clearly identified intraoperatively by matching the corresponding 3D reconstructions.
Hojo et al[21] evaluated the subjective utility of 3D pelvic images and 3D physical models for surgical planning and navigation in LPLND. 3D images were constructed preoperatively from the enhanced CT scan images in 22 patients planned for LPLND for rectal cancer (5 open, 12 Laparoscopic, 5 robotic procedures). The models were printed with white polylactic acid. LPN metastasis was confirmed in 19 sides in 17 patients. Thirty surgeons with experience of laparoscopic colorectal surgery evaluated the subjective usefulness of 3D virtual and printed models by answering a three-item questionnaire using the 5-point Likert scale. The mean score for the subjective usefulness of a 3D model for understanding anatomy was 4.68 (range 3-5) and it was statistically significantly higher in cases with LPN metastases than in those without. Sixty percent of surgeons indicated 3D model, and 27% 3D image as the best modality for preoperative simulation. Eighty-seven percent indicated 3D model, and 13% 3D image as the best modality for intraoperative navigation. 3D models were found to be more helpful for comprehension of 3D spatial anatomy than the virtual models (4.83 and 4.36, respectively, P < 0.001). The ease of use of 3D models and 3D images was scored 4.60 and 4.20, respectively (P = 0.015).
Horie et al[22] reported the application of 3D virtual images in surgical planning and preoperative simulation for laparoscopic LPLND. 3D images were created from CT images and depicted the tumour, branches of the internal mesenteric artery, the iliac artery and vein, ureters, urinary bladder, enlarged lymph nodes, iliopsoas muscle and bones. The records of 10 consecutive patients with advanced low rectal cancer (below peritoneal reflection) who underwent TME, tumour-specific mesorectal resection or total proctocolectomy with LPLND after preoperative 3D simulation were retrospectively reviewed. In four cases (40%) 3D reconstruction revealed variations in vascular anatomy (confirmed intraoperatively), such as duplicate inferior vesical arteries or the obturator artery with a common origin with the internal iliac artery. Authors concluded that 3D preoperative reconstruction can be useful for the safe performance of laparoscopic LPLND.
Recurrent rectal cancer: Hojo et al[23] reported the utility of the 3D virtual and 3D printed models in surgical planning and navigation for the resection of intra-abdominal infra-renal recurrence of colorectal cancer. Amongst eleven patients included in the study, rectum was the site of primary cancer in five, out of which four underwent open and one laparoscopic surgery. 3D virtual images were created preoperatively for nine patients and 3D printed models for two patients. In all patients with rectal cancer virtual models used for intraoperative navigation. R0 resection was achieved in 8 cases. The clinical applicability of this technology was presented in a case of a 65-year-old male with recurrent rectal cancer invading the external iliac artery and vein following low anterior resection. R0 resection of the recurrent tumour together with artificial replacement of both external iliac artery and vein was achieved after the multidisciplinary approach to surgical planning based on 3D virtual model.
Integration of 3D modelling and stereotactic navigation: Nijkamp et al[24] explored the integration of two novel technologies to enhance pelvic cancer surgery. A virtual 3D model of pelvis, including pelvic bones, arteries, veins, and ureters, derived from an enhanced CT scan was integrated into a novel electromagnetic (EM) surgical navigation system for pelvic cancer resections. The 3D model serving as a surgical roadmap was registered to an intraoperative CT scan performed with C-arm cone-beam CT during surgery. The navigation system achieved accuracy of 5 mm and required an additional operating time of 20 min. Thirty-three patients with at least one rigid tumour target were included in the study. Amongst these, seven had a locally advanced primary rectal cancer and one a recurrent rectal cancer with a deposit between external and internal iliac artery. Thirteen surgeons assessed the usability of the tracking system of which 12 completed the questionnaire. The fusion of two novel technologies was found to be feasible. The System Usability Scale score ranged between 57.5 and 95.0 (mean 74), indicating high probability of acceptance.
Surgical education
Normal pelvic anatomy: Hassinger et al[25] presented a pilot study of the usability and perceived effectiveness of a virtual pelvic anatomy simulator (VAPS) – an interactive virtual 3D model created through the segmentation of MR and CT images of a male patient. The interactive 3D model can be manipulated in space, and radiological images were displayed alongside the model. Pelvic structures are labelled with clinically relevant descriptions. All participants (5 medical students and 5 surgical residents) agreed that VAPS teaches clinically relevant anatomy and 90% preferred this type of education to traditional methods. Participants felt that the addition of surgically relevant anatomical details such as Denonvillier’s and Weldeyer’s fascia would be beneficial.
LPLND: Hojo et al[26] conducted a single-centre, open-label, randomised, controlled trial to compare the effectiveness and usefulness of a 3D printed pelvic model as an educational tool for LPLND. Four 3D printed models, previously used for surgical planning of LPLND in patients with rectal cancer and which displayed pelvic bones, ureter, external iliac artery and its branches, obturator nerve and pelvic sidewall muscles, were utilised. The objective utility of 3D models was evaluated with a short and long test. The short test included 10 questions related to pelvic anatomy knowledge. In the long test, participants were asked to name the anatomical structures in the textbook, in virtual 3D images, in 3D printed model and within the intraoperative scene. The subjective utility was assessed through a questionnaire.
A total of 102 participants (34 medical students, 34 residents and 34 junior colorectal surgeons without LPLND experience) were randomly assigned to two groups: the 3D model group and the textbook group. In the first education round, participants studied pelvic anatomy from the 3D model (3D model group) or from the textbook (textbook group). The groups then switched the educational methods. The participants’ knowledge was assessed after each education round. Before education, there was no significant difference in knowledge between the two groups. After education, the short and long test scores of the 3D model group were significantly higher than those of the textbook group for students (short test; P = 0.05, long test; p-0.03), residents (short test; P = 0.05, long test; P = 0.002), and surgeons (short test; P = 0.009, long test; P < 0.001). The questionnaire showed the positive feedback rate to exceed 60%. The rate of positive feedback was lower amongst students than residents and surgeons.
Surgical device design
Brannigan et al[27] applied 3D modelling technology to evaluate the interaction of a standard stapling device with the rectum while dividing it during the TME procedure. Pelvic 3D models were created though semi-automatic segmentation of CT images of six patients planned to undergo elective laparoscopic resection for cancer of the middle and lower third of the rectum. Additionally, a 3D virtual model of a 45º roticulating surgical stapler was created, which allowed for preoperative assessment of the position of the cartridge head in relation to the rectum a simulation tool. The main finding was that with the use of such a stapler, it is physically impossible to achieve perpendicular transection of the rectum. It was shown that to achieve a perpendicular position of the stapler with the mesorectal plane, the stapling device would have to enter the abdomen through right pelvic bone. The standard roticulator with angulation 45º must align with the rectum at an angle of at least 12º. The optimal angulation of the roticulating stapler for transverse rectal stapling would be between 62º and 68º.
DISCUSSION
This systematic review provides an overview of the current applications and the future directions for exploration of the 3D modelling technology in rectal cancer surgery. A small number of eligible studies identified in a thorough literature search and a relatively high proportion of the feasibility or pilot studies indicate that 3D modelling is still in its infancy within the realm of rectal cancer surgery.
TME, which can be performed via open, laparoscopic or robotic approach, has long been established as the gold standard surgical approach to the curative resection of rectal cancer[28,29]. The 3D models, displayed as virtual images[12,25] or physical models[11] can be used to appreciate the spatial pelvic relationships relevant to the TME surgery.
TaTME is a relatively new surgical technique, which was introduced to overcome the inherent limitations of the abdominal approach, such as poor exposure of the TME plane and difficult instrument manipulation in a deep pelvic space[30]. Sahnan et al[14] present the feasibility of construction of a virtual model which can enhance surgical planning and the general comprehension of the TaTME planes. As opposed to the traditional two-dimensional radiological image, a 3D model can be rotated to present the anatomy from the same angle as encountered during surgery. The opacity of the individual components of the model can also be manipulated as required.
MRI is accepted as the gold standard for assessment of rectal cancer[31,32]. The most important prognostic factor from the MR image is the distance of the tumour to the CRM[31]. CRM involvement is associated with an increased risk of local cancer recurrence[33]. Threatened CRM can be reliably assessed on preoperative MRI[34]. However, MRI has been reported to overestimate the CRM involvement in low and anterior tumours[35]. Garcia-Granero et al[16] present a promising novel diagnostic approach to the assessment of the CRM involvement and prostatic infiltration in locally advanced rectal cancer with the mathematic 3D-IPR model which was shown in this feasibility study to have good correlation with the pathology findings.
The same model was demonstrated to be useful in the assessment of infiltration of other surrounding structures in locally advanced primary and recurrent rectal cancer in which case the feasibility of achieving an R0 resection is of paramount significance[17]. In the case presented by the authors 3D-IPR correctly predicted the local infiltration of the ischial bone, which if used for surgical planning, would have allowed for the correct determination of the extent of resection required to achieve R0. The 3D-IPR method may have potential to identify the extent of tumour infiltration more accurately than the MRI images it was derived from but further studies are required to evaluate this.
The management of early rectal cancer presents its own challenges. Almost one-third of screening-detected rectal cancers are confined to the bowel wall without nodal spread[36]. Currently, there is wide variation in management of early rectal cancer but majority proportion of patients are treated with major surgery[37]. However, the minimally invasive approaches, such as transanal excision or transanal minimally invasive surgery are gaining increasing acceptance. High resolution MRI allows for clear depiction of the fine details of the rectal wall and it is possible to distinguish mucosa from the submucosa and the muscularis propria[38]. Przedlacka et al[15] demonstrate the feasibility of segmentation of the rectal wall to illustrate the depth of tumour invasion three-dimensionally. This presents the future direction of the exploration of the role of 3D models as a tool for the assessment of the indication and the extent of local minimally invasive resection.
A separate group of studies describe the role of 3D reconstructions of CT images commonly applied to the assessment of aberrant vascular anatomy or vascular pathologies which if unrecognised, pose a risk of intraoperative bleeding. The 3D virtual[39] and printed[40] models of vascular anatomy relevant to the complete mesocolic excision, particularly when performed with D3 Lymph node dissection, have been shown to be accurate[41] and to improve surgical outcomes, such as operative time, intraoperative blood loss or lymph node harvest[8,42].
In the context on rectal cancer, CT-based 3D images are particularly applicable to the LPLND. Metastasis to the internal iliac and obturator lymph nodes occur in approximately 15% of patients with low rectal cancer[43]. The optimal management of metastatic LPNs is still a subject to a debate with significant differences between the management in Eastern (particularly Japan) and Western countries[44]. Eastern countries tend to adapt a more radical surgical approach with prophylactic LPLND, while Western countries favour the use of neoadjuvant chemoradiotherapy. TME with LPLND is associated with prolonged operative time and potential morbidity, including blood loss and autonomic nerve dysfunction[45]. 3D models can be utilised to assess patient individual vascular anatomy and to locate the metastatic lymph nodes.
Hojo et al[21] demonstrated the subjective usefulness of the 3D models for preoperative planning and intraoperative navigation for LPLND, especially in cases with clinically metastatic LPNs. While large metastatic LPNs are easy to locate intraoperatively, the metastatic LPNs which have reduced in size due to CRT can be more difficult to identify. The use of 3D models derived from the initial staging CT scans obtained prior to the CRT can facilitate locating these nodes. The 3D printed model was perceived superior in this context to the virtual model. The value of 3D printed anatomical models in transferring complex anatomical knowledge has been previously shown by Marconi et al[46].
Novel technologies complement each other in providing a sophisticated environment which enhances surgical vision. Nijkamp et al[24] showed that it was feasible and beneficial to implement virtual 3D models into the stereotactic navigation with a novel EM-tracking system. A similarly promising feasibility for the application of a 3D model in stereotactic navigation for right hemicolectomy was reported by Volonté et al[47]. Optical stereotactic navigation has been previously explored in laparoscopic and robotic locally advanced rectal cancer surgery by Atallah et al[48,49] but in these cases it did not include the use of a 3D reconstructed model. As shown by Brannigan et al[27], 3D modelling technology can also be utilised to guide the development of surgical devices.
Technological advances have revolutionised surgical training as well. It has been shown that computer-based training can enhance acquisition of anatomical and pathological knowledge and that students value highly this approach[49-51]. Due to the low availability, high cost and ethical issues associated with the use of cadavers, traditional cadaver-based training is now largely replaced with simulation or even virtual reality modules[52]. However, as shown by Pellino et al[53] 3D models can equally enhance even the cadaveric simulation. In a patient with a rare retrorectal tumour, a 3D virtual model derived from patient’s radiological images was used for cadaveric simulation of the planned complex procedure with abdominal and perineal approach.
The main factors that contribute to the slow uptake of the 3D modelling technology in rectal cancer surgery are related to the methodology of 3D image generation. 3D models are generated through the segmentation of a two-dimensional radiological image, which can be described as dividing an image into multiple labelled areas representing organs or tissues. Image segmentation relies on the principle that different tissues are characterised by specific range of pixel intensities. It can be performed manually, where each pixel of each slice of the radiological image is labelled manually, semi-automatically or fully automatically, where algorithms that recognise pixel distribution according to a pre-specified threshold are used.
3D modelling has an established role in surgical planning in maxillofacial, orthopaedic and liver surgery[5,6]. Organs, such as bones and muscles, with large contrast between pixel intensities between different tissues on radiological images, lend themselves well to the automatic or semi-automatic segmentation. Radiological MR images of the pelvis require manual segmentation due to close proximity of pixels with similar intensity representing separate organs. This can be extremely labour- and time-consuming. Hamabe and Ito[13] reported time of construction of virtual model of up to 40 h, however, it did significantly decrease with experience.
The ability to reconstruct minute pelvic structures is crucial for the clinical application of 3D modelling[24]. One of the complications of the TME surgery is the autonomic nerve injury leading to impaired urinary and sexual function. This is due to the difficult visualisation of the pelvic plexus, neurovascular bundles and pudendal nerves[54]. It has been shown in the cadaveric and living human studies that it is possible to create 3D representations of the autonomic pelvic nerves, which are at risk of injury during pelvic surgery, from the MRI scans of the cadavers or healthy volunteers, respectively[55,56]. None of the models in the studies reviewed presented pelvic anatomy in such detail.
The potential barrier in the way of clinical application of the 3D printed models in rectal cancer surgery is related to the lack of appropriate material that could replicate the elasticity and plasticity of the bowel wall or fat tissue. Hamabe and Ito[13] noted that technological developments are required before the models suitable for surgical simulation can be fabricated. While cost of 3D printing has been previously cited as another potential barrier, it was not identified as a possible limitation in the present review.
While the feasibility, clinical applicability in selected cases and subjective usefulness of the 3D models in rectal cancer surgery were reported in the studies, their accuracy and the true therapeutic impact of their use in preoperative planning and intraoperative navigation on surgical and oncological outcomes will require further investigation in well-designed randomised controlled studies.
This systematic review has limitations. Firstly, only studies published in English language were included. The level of evidence is low due to the intrinsic studies’ quality. Similarly, owing to the large proportion of feasibility studies, the lack of patients’ demographic information in other studies and heterogenous outcomes reported, no meaningful statistical analysis could be performed.
The future directions of development of the 3D modelling technology in rectal cancer concluded from this review should focus on three main areas – improvement of the 3D modelling technology, validation of the technology and assessment of the benefits and limitations of its application in surgical practice. Firstly, the automation or semi-automation of the segmentation of the two-dimensional radiological image should be sought to reduce the time and workload required for the construction of the 3D model. This can be achieved through the application of the artificial intelligence and machine learning algorithms.
Secondly, the fidelity of 3D models of rectal cancer and pelvis ought to be assessed through well-designed blinded studies validating the prediction of rectal cancer staging provided by the 3D model against the histological assessment of the surgical specimen. Similarly, the accuracy of the patient-specific pelvic anatomical information needs to be validated against the intra-operative findings.
Thirdly, the future randomised controlled studies are required to establish the impact of the application of 3D models on the surgical and oncological outcomes, compared to the established practice of the use of traditional two-dimensional radiological studies in the process of surgical planning. Well-designed multi-centre, randomised trials are required to assess whether there is a statistically significant difference in outcomes, such as surgical time, blood loss, complication rate, R0 resection, CRM, cancer recurrence rate or cancer-free survival, when the use of 3D models and 2D radiological images in operative planning are compared.
The current systematic review identified the need for the future exploration of the application of the 3D models in surgical training. The two examples identified in this review[25,26] indicate a level of interest in this area and show a perceived and objective improvement in anatomical knowledge with the use of 3D models in normal pelvic anatomy and anatomy specifically relevant to LPLND. However, further well-designed randomised controlled studies are needed to establish the impact of the use of the 3D models on the acquisition of pelvic and rectal anatomy understanding, as well as practical surgical skills relevant to the performance of surgical tasks during the rectal cancer surgery, such as TME procedure or minimally invasive rectal cancer approaches.
Lastly, the systematic review revealed the lack of application of 3D modelling technology in patient interaction. The future exploration of this technology needs to also focus on this aspect of the rectal cancer surgical care. It will be necessary to explore the possibility and the impact of the use of 3D models in the process of patient consultation, discussion of the treatment options and obtaining an informed consent.
The future exploration of the 3D modelling technology in rectal cancer surgery should also address the question whether the 3D printed models present any additional benefits compared to the 3D virtual models. This will be relevant to all the fields of application of this technology – surgical planning and operative rehearsal, as well as in the acquisition of the anatomical knowledge or surgical skills, and in patient interaction. In parallel, the technological improvements in the 3D printing materials are required for the construction of clinically relevant 3D printed models and are expected to allow for the creation of physical models, which can more accurately resemble human tissues.
CONCLUSION
The systematic review provides a complete, practical and comprehensive review of the current role of 3D modelling in rectal cancer surgery. It identifies the main areas of interest in this novel approach to patient-tailored image-guided surgery for rectal cancer, and it demonstrates its limitations and directions for the future development and research.
There is an increasing interest in the application of 3D modelling technology in surgical planning and navigation, as well as education, within the realm of rectal cancer surgery. The sixteen studies identified in the review were largely represented by the feasibility or pilot studies, suggesting the relative infancy of the application of this technology in rectal cancer surgery and the need for further research to evaluate its benefits and limitations in clinical practice.
3D modelling can be applied to construct the 3D models, both virtual and physical, of normal pelvic and rectal anatomy, as well as different stages of rectal cancer, including those invading other pelvic structures. 3D models can be applied in surgical planning and navigation in TME, TaTME, beyond-TME surgery or LPLND. They have been showed to improve perceived and objective anatomical knowledge relevant to rectal cancer surgery. However, thus far, 3D models of rectal cancer have not been employed in the patient education or interaction.
Further developments in the 3D modelling methodology and technological developments in 3D printing, as well as future well-designed randomised controlled trials, are necessary for the 3D modelling technology to become clinically applicable in rectal cancer surgery.
ARTICLE HIGHLIGHTS
Research background
Three-dimensional (3D) modelling technology has been gaining an increasing interest in various surgical subspecialities and aspects of surgical care, such as operative planning and navigation, surgical education and patient interaction. However, the uptake of this novel technology lags behind in rectal cancer surgery.
Research motivation
The motivation of the current systematic review is to evaluate the role of 3D modelling technology in rectal cancer surgery and to provide the future directions for its development.
Research objectives
The systemic review aims to provide a comprehensive and up-to-date review of the current applications of 3D modelling technology in rectal cancer surgery and to identify its benefits and limitations.
Research methods
Electronic databases, PubMed/MEDLINE, Embase and Scopus, were searched to identify studies addressing the application of 3D models, both virtual and physical, in rectal cancer surgery between 2000 and 2020. All full-text studies were considered eligible. Animal and cadaveric studies, as well as studies of pelvic volumetry and radiotherapy planning were excluded.
Research results
Sixteen studies were found to be eligible for inclusion in the current systematic review, amongst which there was one single-centre open-label randomised controlled trial, 4 retrospective studies, 9 feasibility or pilot studies and 2 case reports. Thirteen studies described the use of virtual 3D models, one study evaluated 3D printed models and both types of models were described in two studies. The applications of 3D modelling technology in rectal cancer surgery could be divided into four categories: (1) Feasibility of application of 3D modelling technology in rectal cancer surgery; (2) Durgical planning and navigation; (3) Surgical education; and (4) Surgical device design.
Research conclusions
The 3D modelling technology is in its relative infancy in the field of rectal cancer surgery. While the creation of virtual and physical 3D models of rectal cancer and pelvic anatomy has been shown to be feasible, future developments in segmentation technique and 3D printing materials are needed to make it clinically relevant.
Research perspectives
Further well-designed randomised controlled studies are required to assess the fidelity of virtual and physical 3D models of rectal cancer and pelvic anatomy, and to evaluate the influence of their use on surgical and oncological outcomes in rectal cancer surgery.
Footnotes
Conflict-of-interest statement: All the authors declare that they have no competing interests.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Corresponding Author's Membership in Professional Societies: United European Gastroenterology; European Society of Coloproctology ESCP; European Crohn's and Colitis Organisation ECCO; Fellow of the American College of Surgeons FACS; Fellow of the American Society of Colon and Rectal Surgeons FASCRS; Fellow of the Royal College of Surgeons of England FRCS.
Peer-review started: April 28, 2021
First decision: June 17, 2021
Article in press: November 15, 2021
Specialty type: Surgery
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee T S-Editor: Liu M L-Editor: A P-Editor: Liu M
Contributor Information
Anna Przedlacka, Department of Surgery and Cancer, Imperial College London, London SW10 9NH, United Kingdom.
Gianluca Pellino, Department of Advanced Medical and Surgical Sciences, Università degli Studi della Campania “Luigi Vanvitelli”, Naples 80138, Campania, Italy; Colorectal Surgery, Vall d'Hebron University Hospital, Barcelona 08029, Spain; Colorectal Surgery, Royal Marsden NHS Foundation Trust, London SW3 6JJ, United Kingdom. gianluca.pellino@unicampania.it.
Jordan Fletcher, Department of Surgery and Cancer, St Mark’s Hospital Academic Institute, Imperial College London, London HA1 3UJ, United Kingdom.
Fernando Bello, Centre for Engagement and Simulation Science, Imperial College London, London SW10 9NH, United Kingdom.
Paris P Tekkis, Department of Surgery and Cancer, Imperial College London, London SW10 9NH, United Kingdom; Colorectal Surgery, Royal Marsden NHS Foundation Trust, London SW3 6JJ, United Kingdom; Colorectal Surgery, Chelsea and Westminster Hospital NHS Foundation Trust, London SW10 9NH, United Kingdom.
Christos Kontovounisios, Department of Surgery and Cancer, Imperial College London, London SW10 9NH, United Kingdom; Colorectal Surgery, Royal Marsden NHS Foundation Trust, London SW3 6JJ, United Kingdom; Colorectal Surgery, Chelsea and Westminster Hospital NHS Foundation Trust, London SW10 9NH, United Kingdom.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin . 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Maurer CA, Renzulli P, Kull C, Käser SA, Mazzucchelli L, Ulrich A, Büchler MW. The impact of the introduction of total mesorectal excision on local recurrence rate and survival in rectal cancer: long-term results. Ann Surg Oncol . 2011;18:1899–1906. doi: 10.1245/s10434-011-1571-0. [DOI] [PubMed] [Google Scholar]
- 3.Swedish Rectal Cancer Trial, Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE, Wilking N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med . 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 4.Battersby NJ, How P, Moran B, Stelzner S, West NP, Branagan G, Strassburg J, Quirke P, Tekkis P, Pedersen BG, Gudgeon M, Heald B, Brown G MERCURY II Study Group. Prospective Validation of a Low Rectal Cancer Magnetic Resonance Imaging Staging System and Development of a Local Recurrence Risk Stratification Model: The MERCURY II Study. Ann Surg . 2016;263:751–760. doi: 10.1097/SLA.0000000000001193. [DOI] [PubMed] [Google Scholar]
- 5.Hallet J, Gayet B, Tsung A, Wakabayashi G, Pessaux P 2nd International Consensus Conference on Laparoscopic Liver Resection Group. Systematic review of the use of pre-operative simulation and navigation for hepatectomy: current status and future perspectives. J Hepatobiliary Pancreat Sci . 2015;22:353–362. doi: 10.1002/jhbp.220. [DOI] [PubMed] [Google Scholar]
- 6.Morgan C, Khatri C, Hanna SA, Ashrafian H, Sarraf KM. Use of three-dimensional printing in preoperative planning in orthopaedic trauma surgery: A systematic review and meta-analysis. World J Orthop . 2020;11:57–67. doi: 10.5312/wjo.v11.i1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao Y, Wang J, Lv H, Xue Y, Jia R, Liu G, Bai W, Wu Y, Zhang L, Yang J. Diagnostic value of magnetic resonance and computed tomography colonography for the diagnosis of colorectal cancer: A systematic review and meta-analysis. Medicine (Baltimore) . 2019;98:e17187. doi: 10.1097/MD.0000000000017187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bian L, Wu D, Chen Y, Zhang Z, Ni J, Zhang L, Xia J. Clinical Value of Multi-Slice Spiral CT Angiography, Colon Imaging, and Image Fusion in the Preoperative Evaluation of Laparoscopic Complete Mesocolic Excision for Right Colon Cancer: a Prospective Randomized Trial. J Gastrointest Surg . 2020;24:2822–2828. doi: 10.1007/s11605-019-04460-1. [DOI] [PubMed] [Google Scholar]
- 9.Emile SH, Wexner SD. Systematic review of the applications of three-dimensional printing in colorectal surgery. Colorectal Dis . 2019;21:261–269. doi: 10.1111/codi.14480. [DOI] [PubMed] [Google Scholar]
- 10.Papazarkadas X, Spartalis E, Patsouras D, Ioannidis A, Schizas D, Georgiou K, Dimitroulis D, Nikiteas N. The Role of 3D Printing in Colorectal Surgery: Current Evidence and Future Perspectives. In Vivo . 2019;33:297–302. doi: 10.21873/invivo.11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ . 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kontovounisios C, Tekkis P, Bello F. 3D imaging and printing in pelvic colorectal cancer: 'The New Kid on the Block'. Tech Coloproctol . 2019;23:171–173. doi: 10.1007/s10151-018-1922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamabe A, Ito M. A three-dimensional pelvic model made with a three-dimensional printer: applications for laparoscopic surgery to treat rectal cancer. Tech Coloproctol . 2017;21:383–387. doi: 10.1007/s10151-017-1622-z. [DOI] [PubMed] [Google Scholar]
- 14.Sahnan K, Pellino G, Adegbola SO, Tozer PJ, Chandrasinghe P, Miskovic D, Hompes R, Warusavitarne J, Lung PFC. Development of a model of three-dimensional imaging for the preoperative planning of TaTME. Tech Coloproctol . 2018;22:59–63. doi: 10.1007/s10151-017-1724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Przedlacka A, Cox S, Tekkis P, Bello F, Kontovounisios C. Rectal 3D MRI modelling for benign and malignant disease. Br J Surg . 2020;107 e561-e562 [PMID:32841363 DOI: 10.1002/bjs.11858. doi: 10.1002/bjs.11858. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Granero A, Pellino G, Giner F, Frasson M, Fletcher-Sanfeliu D, Romaguera VP, Flor-Lorente B, Gamundi M, Brogi L, Garcia-Calderón D, Gonzalez-Argente FX, Garcia-Granero E. A mathematical 3D-method applied to MRI to evaluate prostatic infiltration in advanced rectal cancer. Tech Coloproctol . 2020;24:605–607. doi: 10.1007/s10151-020-02170-4. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Granero A, Pellino G, Giner F, Frasson M, Fletcher-Sanfeliu D, Primo Romeguera V, Flor Lorente B, Gamundi M, Brogi L, Garcia-Calderón D, González-Argente FX, Garcia-Granero E. A video demonstration of three-dimensional imaging to assess the circumferential resection margin in locally advanced rectal cancer and recurrent rectal cancer - a video vignette. Colorectal Dis . 2020;22:2340–2341. doi: 10.1111/codi.15281. [DOI] [PubMed] [Google Scholar]
- 18.Sueda T, Tei M, Furukawa H, Matsumura T, Koga C, Wakasugi M, Miyagaki H, Kawabata R, Shimizu J, Okada A, Hasegawa J. Surgical treatment of rectal cancer with a Retzius shunt: a case report. Surg Case Rep . 2019;5:25. doi: 10.1186/s40792-019-0583-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen T, Que YT, Zhang YH, Long FY, Li Y, Huang X, Wang YN, Hu YF, Yu J, Li GX. Using Materialise's interactive medical image control system to reconstruct a model of a patient with rectal cancer and situs inversus totalis: A case report. World J Clin Cases . 2020;8:806–814. doi: 10.12998/wjcc.v8.i4.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HJ, Choi GS, Park JS, Park SY, Cho SH, Seo AN, Yoon GS. S122: impact of fluorescence and 3D images to completeness of lateral pelvic node dissection. Surg Endosc . 2020;34:469–476. doi: 10.1007/s00464-019-06830-x. [DOI] [PubMed] [Google Scholar]
- 21.Hojo D, Murono K, Nozawa H, Kawai K, Hata K, Tanaka T, Ishihara S. Utility of a three-dimensional printed pelvic model for lateral pelvic lymph node dissection. Int J Colorectal Dis . 2020;35:905–910. doi: 10.1007/s00384-020-03534-w. [DOI] [PubMed] [Google Scholar]
- 22.Horie H, Koinuma K, Ito H, Sadatomo A, Naoi D, Kono Y, Inoue Y, Morimoto M, Tahara M, Lefor AK, Sata N, Sasaki T, Sugimoto H. Utility of preoperative 3-D simulation of laparoscopic lateral pelvic lymph node dissection for advanced rectal cancer: Surgical outcomes of 10 initial cases. Asian J Endosc Surg . 2018;11:355–361. doi: 10.1111/ases.12476. [DOI] [PubMed] [Google Scholar]
- 23.Hojo D, Emoto S, Kawai K, Nozawa H, Hata K, Tanaka T, Ishihara S. Potential Usefulness of Three-dimensional Navigation Tools for the Resection of Intra-abdominal Recurrence of Colorectal Cancer. J Gastrointest Surg . 2020;24:1682–1685. doi: 10.1007/s11605-020-04626-2. [DOI] [PubMed] [Google Scholar]
- 24.Nijkamp J, Kuhlmann KFD, Ivashchenko O, Pouw B, Hoetjes N, Lindenberg MA, Aalbers AGJ, Beets GL, van Coevorden F, KoK N, Ruers TJM. Prospective study on image-guided navigation surgery for pelvic malignancies. J Surg Oncol . 2019;119:510–517. doi: 10.1002/jso.25351. [DOI] [PubMed] [Google Scholar]
- 25.Hassinger JP, Dozois EJ, Holubar SD, Camp JC, Farley DR, Fidler JL, Pawlina W, Robb RA, Larson DW. Virtual pelvic anatomy simulator: a pilot study of usability and perceived effectiveness. J Surg Res . 2010;161:23–27. doi: 10.1016/j.jss.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Hojo D, Murono K, Nozawa H, Kawai K, Hata K, Tanaka T, Oba K, Ishihara S. Utility of a Three-Dimensional Printed Pelvic Model for Lateral Pelvic Lymph Node Dissection Education: A Randomized Controlled Trial. J Am Coll Surg . 2019;229:552–559.e3. doi: 10.1016/j.jamcollsurg.2019.08.1443. [DOI] [PubMed] [Google Scholar]
- 27.Brannigan AE, De Buck S, Suetens P, Penninckx F, D'Hoore A. Intracorporeal rectal stapling following laparoscopic total mesorectal excision: overcoming a challenge. Surg Endosc . 2006;20:952–955. doi: 10.1007/s00464-005-0536-4. [DOI] [PubMed] [Google Scholar]
- 28.Enker WE. Total mesorectal excision--the new golden standard of surgery for rectal cancer. Ann Med . 1997;29:127–133. doi: 10.3109/07853899709113698. [DOI] [PubMed] [Google Scholar]
- 29.Heald RJ. A new approach to rectal cancer. Br J Hosp Med . 1979;22:277–281. [PubMed] [Google Scholar]
- 30.Sylla P, Rattner DW, Delgado S, Lacy AM. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc . 2010;24:1205–1210. doi: 10.1007/s00464-010-0965-6. [DOI] [PubMed] [Google Scholar]
- 31.Brown G, Daniels IR. Preoperative staging of rectal cancer: the MERCURY research project. Recent Results Cancer Res . 2005;165:58–74. doi: 10.1007/3-540-27449-9_8. [DOI] [PubMed] [Google Scholar]
- 32.Georgiou PA, Tekkis PP, Constantinides VA, Patel U, Goldin RD, Darzi AW, John Nicholls R, Brown G. Diagnostic accuracy and value of magnetic resonance imaging (MRI) in planning exenterative pelvic surgery for advanced colorectal cancer. Eur J Cancer . 2013;49:72–81. doi: 10.1016/j.ejca.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 33.Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR, Sebag-Montefiore D, Tekkis P, Brown G Magnetic Resonance Imaging in Rectal Cancer European Equivalence Study Study Group. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol . 2014;32:34–43. doi: 10.1200/JCO.2012.45.3258. [DOI] [PubMed] [Google Scholar]
- 34.Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg . 2003;90:355–364. doi: 10.1002/bjs.4034. [DOI] [PubMed] [Google Scholar]
- 35.Kim YW, Cha SW, Pyo J, Kim NK, Min BS, Kim MJ, Kim H. Factors related to preoperative assessment of the circumferential resection margin and the extent of mesorectal invasion by magnetic resonance imaging in rectal cancer: a prospective comparison study. World J Surg . 2009;33:1952–1960. doi: 10.1007/s00268-009-0126-z. [DOI] [PubMed] [Google Scholar]
- 36.Greenaway K HJ, Khatun S. National Bowel Cancer Audit Report 2015. [cited 20 March 2021]. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/national-bowel-cancer-audit/national-bowel-cancer-audit-report-2015 .
- 37.Balyasnikova S, Brown G. The MRI assessment of SPECC (significant polyps and early colorectal cancer) lesions. Colorectal Dis . 2019;21 Suppl 1:19–22. doi: 10.1111/codi.14526. [DOI] [PubMed] [Google Scholar]
- 38.Brown G, Kirkham A, Williams GT, Bourne M, Radcliffe AG, Sayman J, Newell R, Sinnatamby C, Heald RJ. High-resolution MRI of the anatomy important in total mesorectal excision of the rectum. AJR Am J Roentgenol . 2004;182:431–439. doi: 10.2214/ajr.182.2.1820431. [DOI] [PubMed] [Google Scholar]
- 39.Miyamoto R, Tadano S, Sano N, Inagawa S, Adachi S, Yamamoto M. The impact of three-dimensional reconstruction on laparoscopic-assisted surgery for right-sided colon cancer. Wideochir Inne Tech Maloinwazyjne . 2017;12:251–256. doi: 10.5114/wiitm.2017.67996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Granero A, Sánchez-Guillén L, Fletcher-Sanfeliu D, Flor-Lorente B, Frasson M, Sancho Muriel J, Alvarez Serrado E, Pellino G, Grifo Albalat I, Giner F, Roca Estelles MJ, Esclapez Valero P, Garcia-Granero E. Application of three-dimensional printing in laparoscopic dissection to facilitate D3-lymphadenectomy for right colon cancer. Tech Coloproctol . 2018;22:129–133. doi: 10.1007/s10151-018-1746-9. [DOI] [PubMed] [Google Scholar]
- 41.Hiroishi A, Yamada T, Morimoto T, Horikoshi K, Nakajima Y. Three-dimensional computed tomographic angiography with computed tomographic colonography for laparoscopic colorectal surgery. Jpn J Radiol . 2018;36:698–705. doi: 10.1007/s11604-018-0775-7. [DOI] [PubMed] [Google Scholar]
- 42.Nesgaard JM, Stimec BV, Bakka AO, Edwin B, Ignjatovic D RCC study group. Navigating the mesentery: a comparative pre- and per-operative visualization of the vascular anatomy. Colorectal Dis . 2015;17:810–818. doi: 10.1111/codi.13003. [DOI] [PubMed] [Google Scholar]
- 43.Akiyoshi T, Watanabe T, Ueno M. Is lateral pelvic lymph node dissection no longer necessary for low rectal cancer after neoadjuvant therapy and TME to reduce local recurrence? J Gastrointest Surg . 2012;16:2341–2342. doi: 10.1007/s11605-012-1955-x. [DOI] [PubMed] [Google Scholar]
- 44.Malakorn S, Chang GJ. Treatment of rectal cancer in the East and West: Should it be different? Surgery . 2017;162:315–316. doi: 10.1016/j.surg.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Georgiou P, Tan E, Gouvas N, Antoniou A, Brown G, Nicholls RJ, Tekkis P. Extended lymphadenectomy vs conventional surgery for rectal cancer: a meta-analysis. Lancet Oncol . 2009;10:1053–1062. doi: 10.1016/S1470-2045(09)70224-4. [DOI] [PubMed] [Google Scholar]
- 46.Marconi S, Pugliese L, Botti M, Peri A, Cavazzi E, Latteri S, Auricchio F, Pietrabissa A. Value of 3D printing for the comprehension of surgical anatomy. Surg Endosc . 2017;31:4102–4110. doi: 10.1007/s00464-017-5457-5. [DOI] [PubMed] [Google Scholar]
- 47.Volonté F, Pugin F, Buchs NC, Spaltenstein J, Hagen M, Ratib O, Morel P. Console-integrated stereoscopic OsiriX 3D volume-rendered images for da Vinci colorectal robotic surgery. Surg Innov . 2013;20:158–163. doi: 10.1177/1553350612446353. [DOI] [PubMed] [Google Scholar]
- 48.Atallah S, Larach SW, Monson JR. Stereotactic navigation for TAMIS-TME. Minim Invasive Ther Allied Technol . 2016;25:271–277. doi: 10.1080/13645706.2016.1201119. [DOI] [PubMed] [Google Scholar]
- 49.Atallah S, Parra-Davila E, Melani AGF, Romagnolo LG, Larach SW, Marescaux J. Robotic-assisted stereotactic real-time navigation: initial clinical experience and feasibility for rectal cancer surgery. Tech Coloproctol . 2019;23:53–63. doi: 10.1007/s10151-018-1914-y. [DOI] [PubMed] [Google Scholar]
- 50.Lin C, Gao J, Zheng H, Zhao J, Yang H, Lin G, Li H, Pan H, Liao Q, Zhao Y. Three-Dimensional Visualization Technology Used in Pancreatic Surgery: a Valuable Tool for Surgical Trainees. J Gastrointest Surg . 2020;24:866–873. doi: 10.1007/s11605-019-04214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeo CT, MacDonald A, Ungi T, Lasso A, Jalink D, Zevin B, Fichtinger G, Nanji S. Utility of 3D Reconstruction of 2D Liver Computed Tomography/Magnetic Resonance Images as a Surgical Planning Tool for Residents in Liver Resection Surgery. J Surg Educ . 2018;75:792–797. doi: 10.1016/j.jsurg.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 52.Rosen KR. The history of medical simulation. J Crit Care . 2008;23:157–166. doi: 10.1016/j.jcrc.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Pellino G, García-Granero A, Fletcher-Sanfeliu D, Navasquillo-Tamarit M, Frasson M, García-Calderon D, García-Gausi M, Valverde-Navarro AA, Garcia-Armengol J, Roig-Vila JV, García-Granero E. Preoperative surgical planning based on cadaver simulation and 3D imaging for a retrorectal tumour: description and video demonstration. Tech Coloproctol . 2018;22:709–713. doi: 10.1007/s10151-018-1854-6. [DOI] [PubMed] [Google Scholar]
- 54.Wallner C, Lange MM, Bonsing BA, Maas CP, Wallace CN, Dabhoiwala NF, Rutten HJ, Lamers WH, Deruiter MC, van de Velde CJ Cooperative Clinical Investigators of the Dutch Total Mesorectal Excision Trial. Causes of fecal and urinary incontinence after total mesorectal excision for rectal cancer based on cadaveric surgery: a study from the Cooperative Clinical Investigators of the Dutch total mesorectal excision trial. J Clin Oncol . 2008;26:4466–4472. doi: 10.1200/JCO.2008.17.3062. [DOI] [PubMed] [Google Scholar]
- 55.Bertrand MM, Macri F, Mazars R, Droupy S, Beregi JP, Prudhomme M. MRI-based 3D pelvic autonomous innervation: a first step towards image-guided pelvic surgery. Eur Radiol . 2014;24:1989–1997. doi: 10.1007/s00330-014-3211-0. [DOI] [PubMed] [Google Scholar]
- 56.Wijsmuller AR, Giraudeau C, Leroy J, Kleinrensink GJ, Rociu E, Romagnolo LG, Melani AGF, Agnus V, Diana M, Soler L, Dallemagne B, Marescaux J, Mutter D. A step towards stereotactic navigation during pelvic surgery: 3D nerve topography. Surg Endosc . 2018;32:3582–3591. doi: 10.1007/s00464-018-6086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


