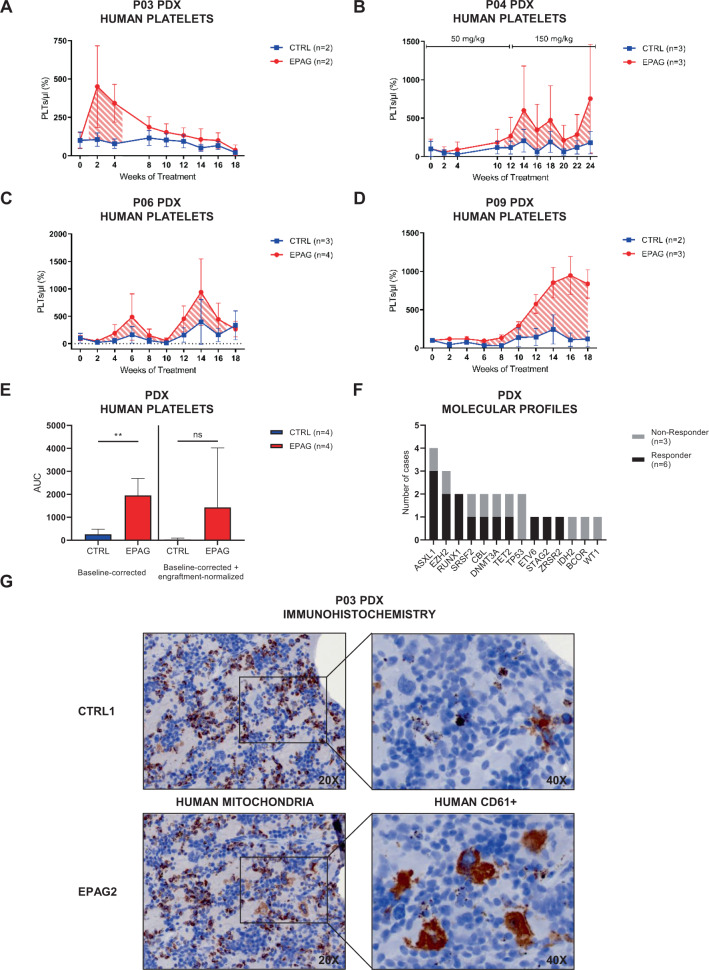
Fig. 3. Increased thrombo- and megakaryopoiesis in EPAG-treated MDS patient-derived xenografts.
A–D Mean baseline-corrected number of human platelets (PLTs) in the course of 18–24 weeks of treatment for groups of eltrombopag- (EPAG, red) and vehicle-treated (CTRL, blue) xenografted mice of P03, P04, P06, and P09. Striped areas indicate response to EPAG defined as two-fold change of PLT production. Initial number of PLTs for each mouse was taken as individual baseline. Peripheral blood (PB) was sampled biweekly, unless otherwise indicated. The absolute number of human PLTs per microliter PB was calculated in relation to the number of beads recorded. For P04, dose was escalated after 12 weeks from 50 to 150 mg/kg for additional 12 weeks. See also Supplementary Fig. S4A, B for data on additional cases. E Area under the curve (AUC) values for graphs A–D and mean baseline-corrected engraftment-normalized data (not shown). Data were analyzed using unpaired, two-tailed t-test. F Comparison of MDS-related mutations between xenograft non-responder and responder. See also Supplementary Fig. S4H for data on cytogenetic aberrations. G Serial paraffin sections from CTRL1 and EPAG2 of P03 were stained for human mitochondria and CD45+ CD61+ megakaryocytes using immunohistochemistry (magnification ×20 and ×40). Data in A–E are represented as mean ± SD. **p < 0.01.