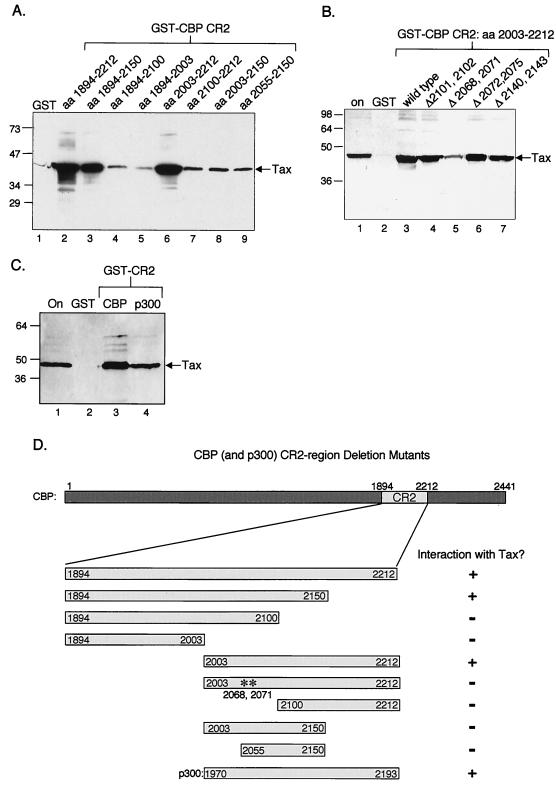
FIG. 2.
Identification and characterization of minimal CR2 domain. (A) Tax interacts with aa 2003 to 2212 of CBP in vitro. Purified recombinant Tax (50 pmol) was incubated with GST alone or the indicated GST-CR2 deletion mutants (50 pmol). As a positive control, Tax binding to the full-length region of GST-CR2aa1894–2212 was also tested. The bound proteins were separated by SDS–12% PAGE, transferred to nitrocellulose, and detected using an anti-His6 antibody. Positions of bound Tax and protein molecular size standards are indicated (in kilodaltons). The Western blot was stripped and reprobed with anti-GST to ensure that equal amounts of GST fusion protein were used in the assay. (B) Tax is defective for interaction with the double point mutant ΔCR2 L2068→A/L2071→A. Purified recombinant Tax (50 pmol) was assayed for its ability to bind to GST alone or the GST-CR2aa2003–2212 double point mutants F2101→A/I2102→A, L2068→A/L2071→A, L2072→A/L2075→A, and L2140→A/L2143→A (50 pmol each). Tax binding to wild-type GST-CR2aa2003–2212 was tested as a positive control. The bound proteins were separated by SDS–12% PAGE, transferred to nitrocellulose, and detected using an anti-His6 antibody. Bound Tax and protein molecular size standards are indicated (in kilodaltons). Five percent of the Tax onput is shown in lane 1. (C) Tax binds equally well to the CR2 domains derived from CBP and p300. Purified recombinant Tax (50 pmol) was incubated with GST alone or the GST-CR2 region from CBP (aa 2003 to 2212) or p300 (aa 1970 to 2193) (50 pmol each). The bound proteins were electrophoresed on SDS–12% PAGE, transferred to nitrocellulose, and detected using an anti-His6 antibody. Bound Tax and protein molecular size standards are indicated. Asterisks indicate the double point mutant (in kilodaltons) in GST-ΔCR2 L2068→A/L2071→A. Bound Tax was quantified using ImageQuant, and the results indicated that the intensities were nearly equal. (D) Summary of CR2-Tax interactions.