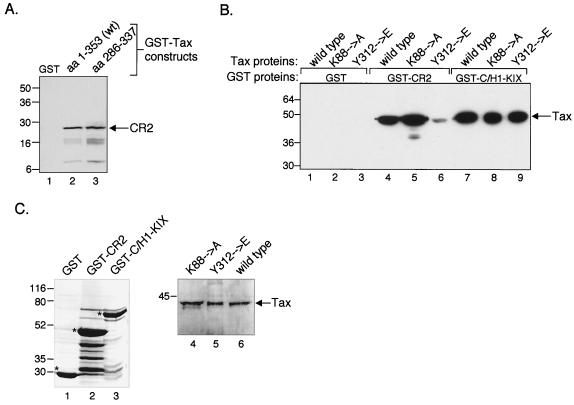
FIG. 4.
Carboxy-terminal activation domain of Tax interacts with CR2. (A) CR2 binds to the putative transactivation domain of Tax. Purified, recombinant His6-CR2aa2003–2212 (50 pmol) was incubated with GST alone or the indicated GST-Tax fusion constructs (50 pmol). The bound proteins were separated by SDS–10% PAGE, transferred to nitrocellulose, and detected using an anti-His6 antibody. The positions of bound His6-CR2aa2003–2212 and protein molecular size standards are indicated (in kilodaltons). (B) The Tax Y312→E point mutant is defective for interaction with CR2. Purified recombinant wild-type Tax (lanes 1, 4, and 7), Tax K88→A (lanes 2, 5, and 8), or Tax Y312→E (lanes 3, 6, and 9) (50 pmol each) were incubated with GST alone or the indicated GST-CR2aa2003–2212 or GST-C/H1-KIXaa302–683 fusion proteins. The bound proteins were separated by SDS–12% PAGE, transferred to nitrocellulose, and detected using an anti-His6 antibody. Bound Tax and protein molecular size standards are indicated (in kilodaltons). (C) Coomassie-stained gels of the purified proteins used in the experiment shown in panel B. The left panel shows the GST fusion proteins; asterisks denote the relevant protein band. The right panel shows both the wild-type and mutant forms of Tax used in the experiment. Protein molecular size standards are indicated (in kilodaltons).