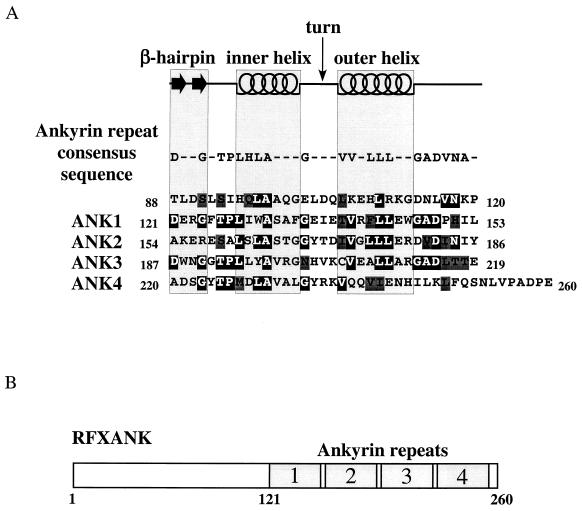
FIG. 1.
RFXANK contains four ankyrin repeats. (A) Sequence analysis of the ankyrin repeat domain of RFXANK. The secondary-structure elements (β-hairpin loops, inner helix, turn, and outer helix) and the ankyrin repeat consensus sequence are displayed above the amino acid sequence of RFXANK residues 88 to 260. Identical and conserved residues relative to the ankyrin repeat consensus sequence are represented by white letters on a black background and by black letters on a dark gray background, respectively. A high degree of sequence similarity to the ankyrin consensus motif sequence can be observed from amino acid V117 to I242, suggesting the formation of four ankyrin repeats in RFXANK. (B) Schematic representation of RFXANK. RFXANK contains 260 amino acid residues and four ankyrin repeats at the C-terminal part of the protein.