Figure 2.
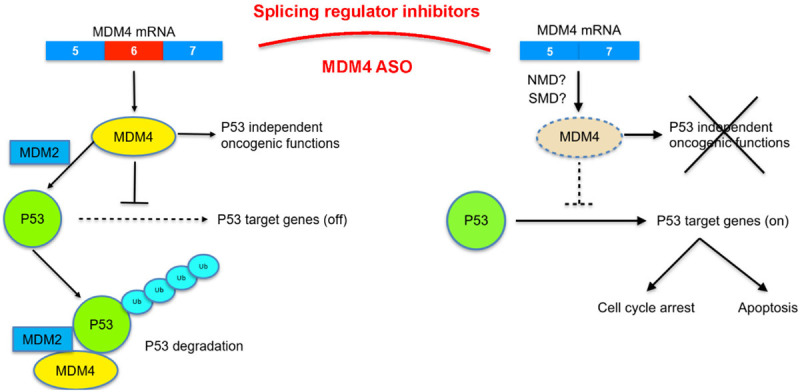
Targeting MDM4 splicing in cancer therapy. In normal adult tissues, exon6 of MDM4 tends to be skipped due to unproductive splicing. This will produce unstable transcript which is prone to be degraded through NMD or SMD pathway and decrease the abundance of MDM4-FL protein, which further transactivates and represses a number of target genes that function in apoptosis, cell cycle, and DNA repair (right part). While MDMX protein is highly expressed in embryonic tissues and in cancers due to the enhanced exon 6 inclusion. MDM4 can work together with MDM2 to mediate p53 degradation and perform both p53-dependent and independent oncogenic functions. Current strategy is that splicing regulator inhibitors (such as pladienoide b or GSK591) or MDM4 ASO can be used to induce MDM4 skipping to decrease the MDM4 abundance, and further inhibit p53-dependent or independent MDM4 oncogenic functions. Either splicing regulator inhibitors or antisense oligonucleotides (ASOs) are very specific, efficient, and clinically compatible approach.
