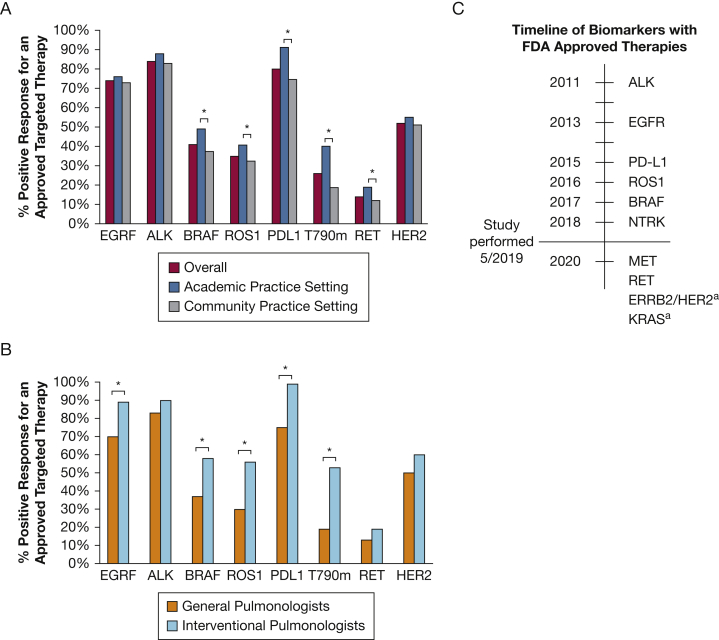
Figure 4.
A, Percent responding that an FDA-approved targeted therapy exists for the corresponding molecular biomarker for all respondents, and by academic and community practice settings. B, Percent responding that an FDA-approved targeted therapy exists for the corresponding molecular biomarker comparing general and interventional pulmonologists. C, Timeline of FDA approval for molecular biomarkers with associated targeted therapeutics. aFDA breakthrough therapy designation only (not full approval). FDA = Food and Drug Administration. b∗P < .05.