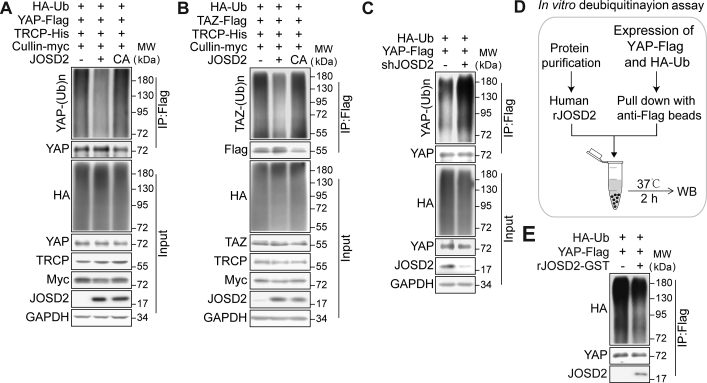
Figure 4.
JOSD2 removes the poly-ubiquitin chains on YAP/TAZ. (A, B) JOSD2 decreases ubiquitination of YAP (A) and TAZ (B) in a catalytic activity-dependent manner. JOSD2-WT or JOSD2-C24A, HA-tagged ubiquitin and flag-tagged YAP or TAZ were co-expressed into 293T cells in the presence of β-TRCP and cullin1 (known as E3 ligase of YAP/TAZ), then the cells were treated with MG132 (10 μmol/L) for 6 h before harvest. Total cell lysates were immune-precipitated with anti-DYKDDDDK IP resin to detect the poly-ubiquitin chains on YAP/TAZ. (C) Depletion of JOSD2 increases YAP ubiquitination. HA-tagged ubiquitin and flag-tagged YAP plasmids were co-transfected into 293T cells with or without JOSD2 depletion followed by MG132 (10 μmol/L, 6 h) treatment. Cell lysates were immune-precipitated with anti-DYKDDDDK IP resin and subjected to immunoblotting analysis. (D) and (E) Bacterial-expressed recombinant human JOSD2 (rhJOSD2) effectively removes the poly-ubiquitination on YAP in vitro. 293T cells transfected with HA-tagged ubiquitin and Flag-tagged YAP were lysed and the ubiquitinated YAP was pulled down by anti-DYKDDDDK IP resin to incubate with purified rhJOSD2 for 2 h at 37 °C. Subsequently, immunoblotting was performed to assess YAP ubiquitination level.