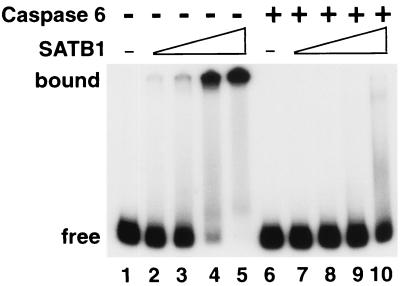
FIG. 6.
Cleavage by caspase 6 abolishes the DNA-binding activity of SATB1. Wild-type SATB1 was transcribed and translated in vitro and digested with recombinant activated caspase 6 as described in Materials and Methods. 32P-labeled BUR probe WT (25)7 was prepared as described previously (37). The binding reactions were performed as described in Materials and Methods and then resolved by 6% native PAGE. Free, position of the labeled DNA substrate alone (lanes 1 and 6). Mock-treated (lanes 2 to 5) and caspase 6-treated (lanes 7 to 10) in vitro translation mixtures were serially diluted in EMSA buffer and incubated with the labeled WT (25)7-mer. One microliter of either 20-fold-diluted (lanes 2 and 7), 10-fold-diluted (lanes 3 and 8), or 5-fold-diluted (lanes 4 and 9) or undiluted (lanes 5 and 10) translation mixture was used for each binding reaction. The positions of SATB1-DNA complexes (bound) and the free DNA probe (free) are indicated on the left.