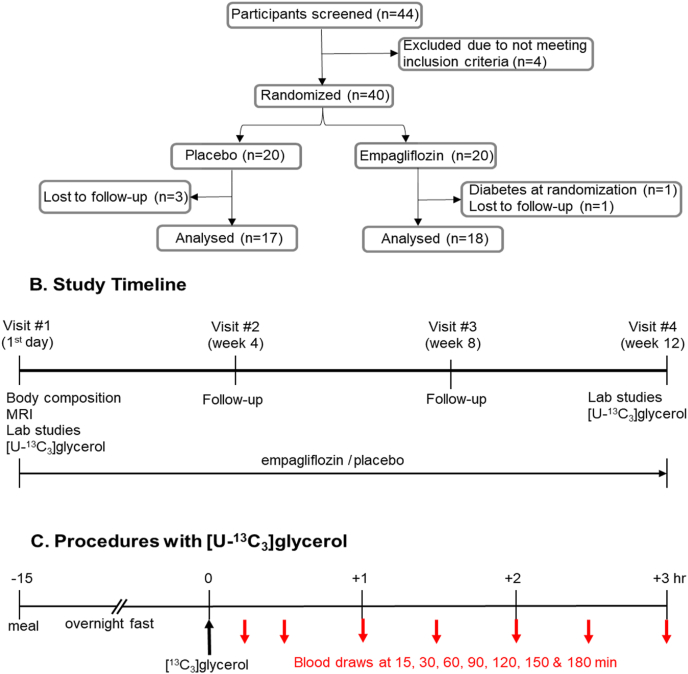
Fig. 1.
Study procedures. (A) Participants (n = 40) were randomly assigned to either placebo or empagliflozin. Three subjects from the placebo group and two from the empagliflozin group dropped out, and 17 volunteers in the placebo group and 18 in the empagliflozin group completed the study. (B) Participants made four visits with 4-week intervals. At the first visit after an overnight fast, MRI was performed for body composition measurement. Blood was drawn for biochemical analysis, and procedures with [U–13C3]glycerol were performed. Participants were assigned to either placebo or empagliflozin. At the second and the third follow-up visits, drug adherance and side effects were monitored. At the last visit after an overnight fast, blood was drawn for analysis and the procedures with [U–13C3]glycerol were performed. (C) At the first and the fourth visits, participants drank [U–13C3]glycerol-dissolved water and blood was drawn at multiple time points (15, 30, 60, 90, 120, 150 and 180 min) for NMR analysis of triglycerides.