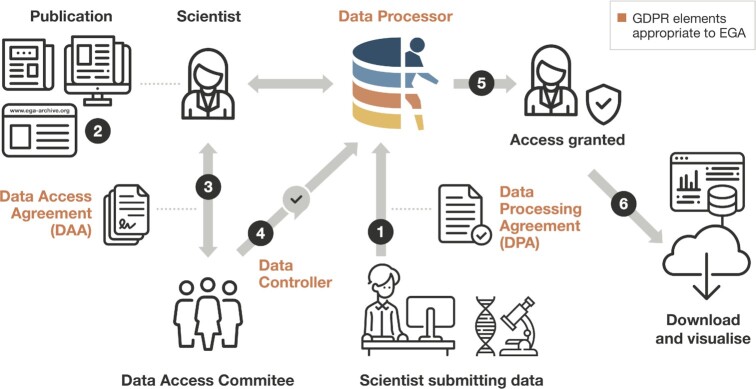
Figure 2.
EGA facilitates the submission, discovery, access, and distribution of sensitive human data. A researcher submits controlled access human genetic, phenotypic and clinical data to EGA after signing a Data Processing Agreement (1). EGA processes, archives, and releases the dataset to be findable. Another researcher discovers data of interest at the EGA (2). They contact the Data Access Committee for the data of interest and agree to the terms of data reuse by signing a Data Access Agreement (3). The Data Access Committee informs EGA that access is approved (4). The EGA grants access to the requesting researcher (5) who can then download and visualise the data (6). GDPR: General Data Protection Regulation.