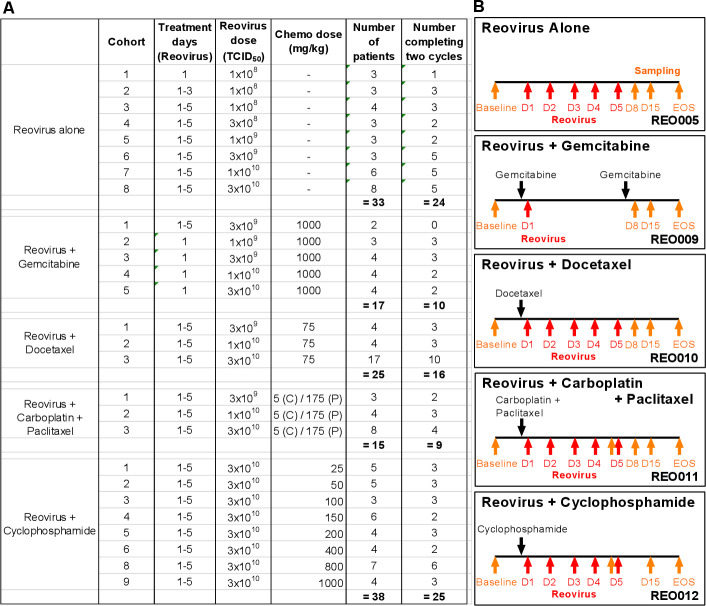
Figure 1.
Doses and treatment schedules for phase I studies of reovirus and chemotherapy. (A) Clinical trials include reovirus (REO) administered intravenously as a single-agent (REO005) or in combination with chemotherapy: gemcitabine (REO009), docetaxel (REO010), carboplatin and paclitaxel (REO011), and cyclophosphamide (REO012). With the exception of REO009, reovirus was given consecutively for the first 5 days of the treatment cycle. For REO005 and REO012, each cycle lasted 28 days, and for REO009, REO010 and REO011, each cycle lasted 21 days. (B) Blood samples for antibody analysis were collected as indicated by the orange arrows. Reovirus was administered within the first 5 days (days 1–5) of the treatment cycle as indicated by the red arrows. EOS, end of study.