Fig. 3.
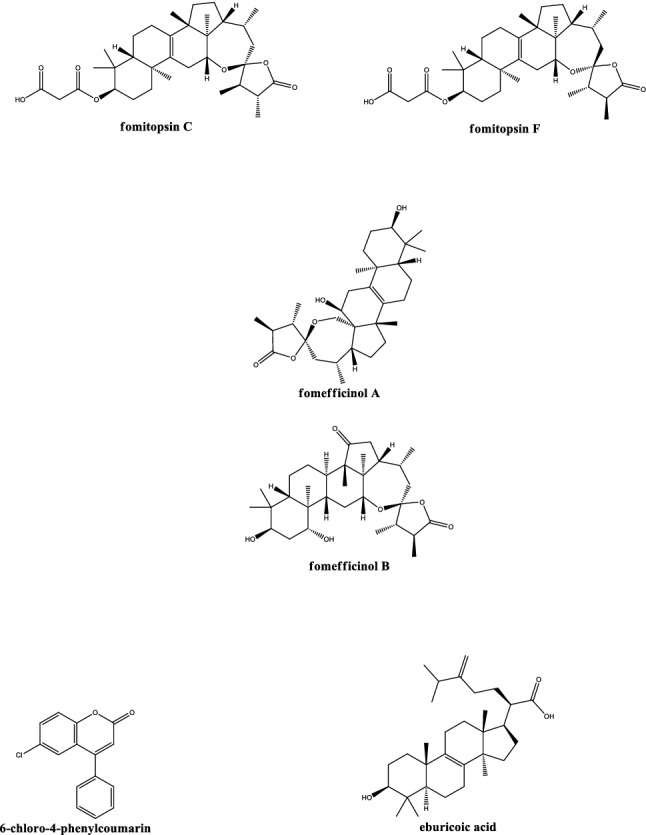
Chemical structures of some phytochemicals isolated from Fomitopsis officinalis. Note how fomitopsin C and F, as well fomefficinol A and B were specifically identified from extracts of the Fomitopsis officinalis. Other isolated compounds such as eburicoic acid and 6-chloro-4-phenylcoumarin are endowed with anti-inflammatory and antimicrobial properties. Chemical use names (bold) with the corresponding IUPAC names: fomitopsin C 3-[(1S,3'R,4'R,5R,7R,10S,13R,15S,17R,18R,21R)-1,3',4',6,6,10,17,21-octamethyl-5'-oxospiro[14-oxapentacyclo[11.7.1.02,11.05,10.018,21]henicos-2(11)-ene-15,2'-oxolane]-7-yl] oxy-3-oxopropanoic acid; fomitopsin F 3-[(1S,3'S,4'S,5R,7R, 10S,13R,15R,17R,18R,21R)-1,3',4',6,6,10,17,21-octamethyl-5'-oxospiro[14-oxapentacyclo[11.7.1.02,11.05,10.018,21]henicos-2(11)-ene-15,2'-oxolane]-7-yl]oxy-3-oxopropanoic acid; fomefficinol A (1R,2S,3'S,4'S,5S,8R,10R,14S,17R,18R,20S)-2,8-dihydroxy-3',4',5,9,9,14,18-heptamethylspiro[21-oxapentacyclo[12.8.0.01,17.04,13.05,10]docos-4(13)-ene-20,5'-oxolane]-2'-one; fomefficinol B (1S,2R,3'S,4'S,5S,7R,9R,10R,11S,13R,15S,17R,18R,21R)-7,9-dihydroxy-1,3',4',6,6,10,17,21-octamethylspiro[14-oxapentacyclo[11.7.1.02,11.05,10.018,21]henicosane-15,5'-oxolane]-2',20-dione; 6-chloro-4-phenylcoumarin 6-chloro-4-phenyl-2H-chromen-2-one; eburicoic acid (R)-2-((3S,5R,10S,13R,14R,17R)-3-hydroxy-4,4,10,13,14-pentamethyl-2,3,4,5,6,7,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)-6-methyl-5-methyleneheptanoic acid
