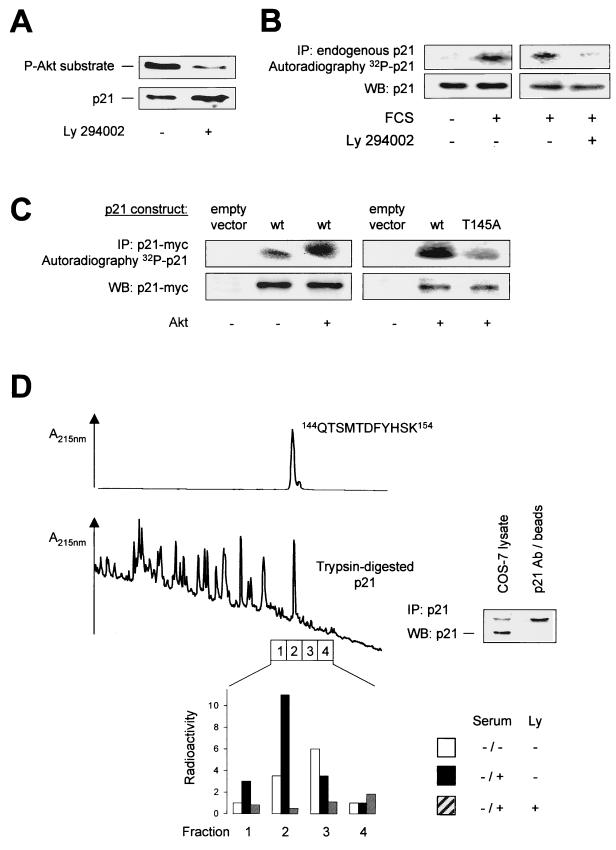
FIG. 3.
In vivo phosphorylation of p21Cip1. (A) Detection of phosphorylated p21Cip1 by immunoblot analysis of HUVEC extracts using a phospho-specific antibody against the Akt phosphorylation consensus motif. Right lane, effect of Ly294002 (10 μM) for 1 h before lysis. The lower panel shows total endogenous p21Cip1 for comparison. (B) In vivo phosphorylation of p21Cip1 by a serum-induced, PI3K-sensitive mechanism. HUVEC were labeled with 32P and starved for 1 h in FCS-free medium before the addition of 10% phosphate-free FCS and Ly294002 (10 μM) for 30 min as indicated. Endogenous p21Cip1 was immunoprecipitated (IP) with anti-p21Cip1 antibodies. (C). In vivo phosphorylation of p21Cip1 by Akt. COS-7 cells overexpressing myc-tagged p21Cip1 constructs and vector (pcDNA3.1) or Akt constructs were labeled with 32P for 3 h, and p21Cip1 was immunoprecipitated with anti-myc antibodies. In panels B and C, representative autoradiographs are shown: lower panels, expression of p21Cip1 as a loading control. (D) Phospho-peptide analysis. (Top) Elution of the synthetic peptide QTSMTDFYHSK (where T is the phospho-acceptor amino acid) corresponding to amino acids 144 to 154 of p21 from the reverse phase column. This peptide contains the putative Akt phosphorylation site (145Thr) in p21 and would result from tryptic digestion of p21. (Middle) Endogenous p21 was immunoprecipitated from COS cells, and tryptic p21 peptides were separated by reverse-phase chromatography. Note the peak corresponding to the peptide QTSMTDFYHSK. (Bottom) Following serum starvation, COS cells were serum treated in the presence of 32P with or without Ly294002 (10 μM). Tryptic p21 peptides were seperated as above, and fractions surrounding peptide QTSMTDFYHSK were collected for determination of radioactivity. A representative result is shown. WB, Western blotting.