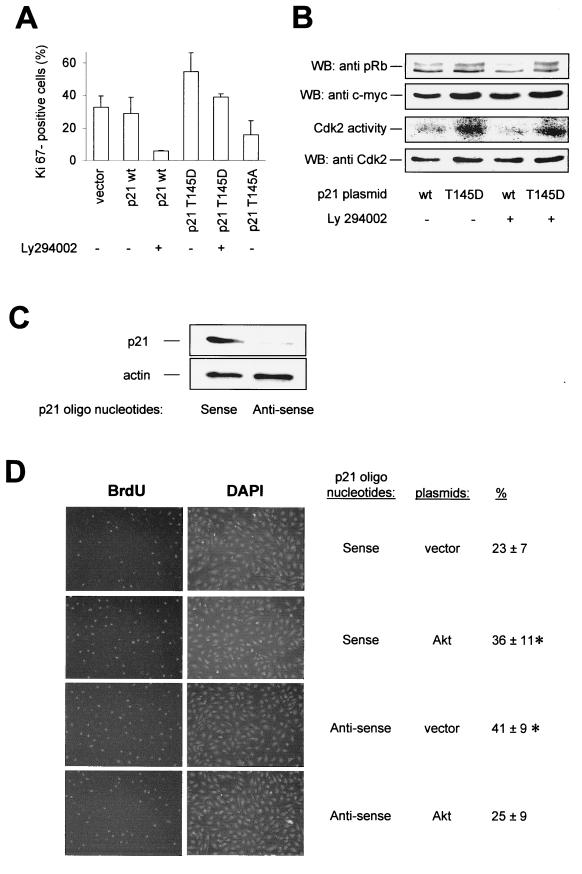
FIG. 9.
Effect of p21Cip1 Thr 145 phosphorylation on endothelial cell proliferation. (A) HUVEC were cotransfected with GFP (1 μg) and the respective pcDNA3.1 constructs (2 μg) and incubated for 20 h. Then, cells were treated with Ly294002 (10 μM) for 12 h. GFP-positive cells were isolated by FACS, and proliferative endothelial cells were identified by immunostaining against the proliferative marker protein Ki67 (data are mean ± SEM; n = 3 to 4). (B) (Top) Western blot analysis (WB) of pRb phosphorylation in intact cells; the upper band corresponds to hyperphosphorylated pRb and the lower band corresponds to hypophosphorylated pRb in HUVEC overexpressing p21 wt or T145D in the absence or presence of Ly294002. In the second panel from the top, expression of p21Cip1 constructs is shown; in the third panel, an autoradiograph of in vitro-phosphorylated pRb in a Cdk2 kinase assay is shown. The bottom panel shows a Western blot of Cdk2 immunoprecipitates from the respective lysates. (C) HUVEC were transfected with p21Cip1 antisense or sense oligonucleotides, and p21Cip1 protein expression was detected by Western blot analysis using an antibody against endogenous p21Cip1. A representative blot of three individual experiments is shown. (D) Effect of Akt in p21Cip1 antisense-transfected HUVEC that were cotransfected with p21Cip1 antisense or sense oligonucleotides, GFP, and active Akt (T308D/S473D) or vector. Eighteen hours after transfection, the proliferation of GFP-positive cells was assessed by BrdU staining, followed by counterstaining with DAPI. Representative photomicrographs are shown (data are mean ± SEM, n = 3; P < 0.05 versus p21 sense plus vector).