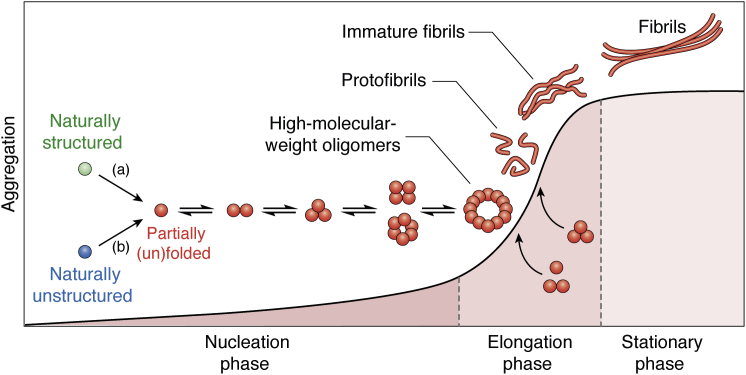
Figure 1.
A Schematic diagram of amyloidogenic proteins aggregation. Amyloidogenic proteins can be naturally structured or unstructured. Naturally structured proteins (a), e.g., PrP undergo partial unfolding, whereas naturally unstructured proteins (b), such as Aβ, αSyn, or tau, undergo partial folding under pathological conditions, initiating the self-assembly process. Both cases lead to formation of partially (un)folded monomers, which self-associate into increasing-size oligomers until a quasi-stable nucleus forms leading to the elongation phase. Elongation typically proceeds at a fast rate compared with the nucleation and may involve formation of quasi-stable high-molecular-weight oligomers, protofibrils, and eventually fibrils. Finally, the monomers are consumed and the system reaches a stationary phase in which no more growth is observed.