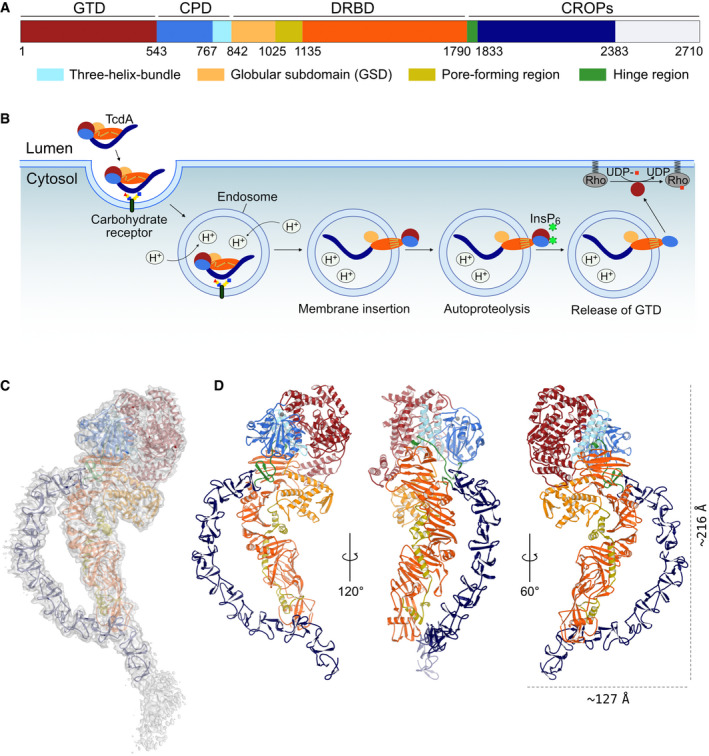
A schematic representation of TcdA, which shows the domain organization including identified subdomains and functional regions. GTD, glycosyltransferase domain (dark red); CPD, cysteine protease domain (blue) containing the three‐helix bundle (3HB) (light blue); DRBD, delivery and receptor‐binding domain (orange) containing the globular subdomain (GSD) (yellow), the pore‐forming region (olive), and the hinge region (green); CROPs, combined repetitive oligopeptides (dark blue). The white area of the C‐terminal part of the CROPs domain represents a part of the structure that could not be modeled in the TcdA structure due to deteriorating cryo‐EM map quality.
The multistep mechanism of intoxication. Various carbohydrate moieties (and gp96 or LPLR) are receptors for TcdA. The toxin is internalized by endocytosis and acidification of the endosome, which triggers a conformational change in the DRBD and results in pore formation and translocation of the GTD, and likely also CPD, into the cytosol. Next, inositol hexakisphosphate (InsP6) binds and activates the CPD which cleaves and releases the GTD. The GTD translocates to the cell membrane and glycosylates the Rho family of GTPases causing pathogenic cell rounding and apoptotic cell death.
Cryo‐EM map of native TcdA contoured at 7 σ with a cartoon representation of the structure placed inside. The individual domains are colored as in panel A.
Cartoon representation of TcdA from three different angles with zinc shown as a gray sphere.