-
A
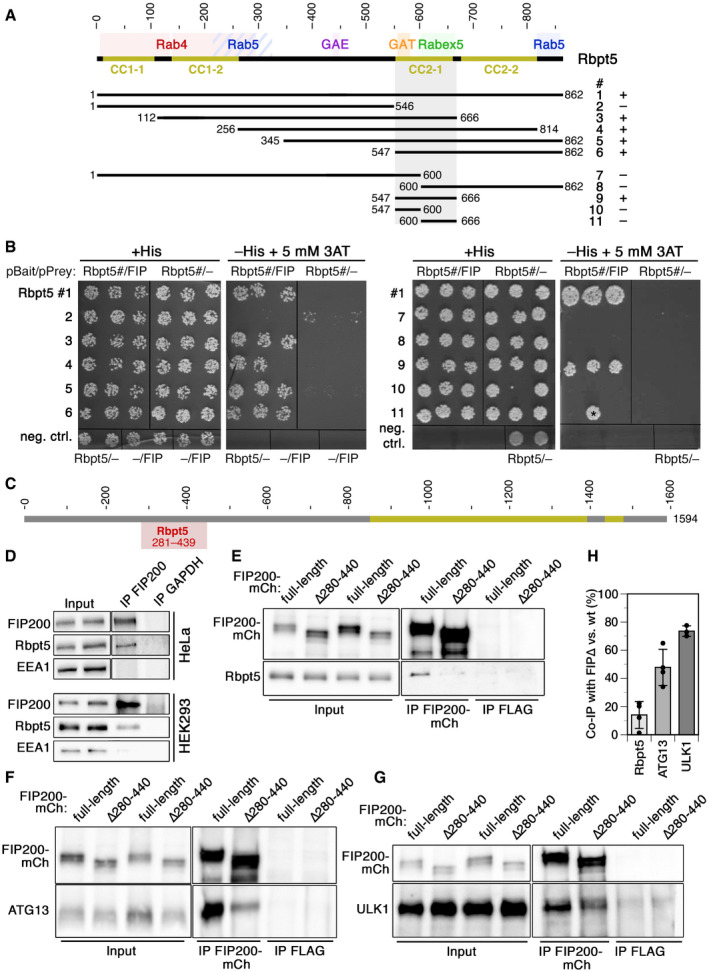
Schematic representation of the sequence of Rabaptin5. Coiled‐coil (CC) segments are shown in yellow. Colored backgrounds highlight the segments shown to interact with Rab4, Rab5, Rabex5, and the GAE and GAT domains of GGAs (Golgi‐localizing, γ‐adaptin ear homology domain, ARF‐binding proteins). Below, the segments used to test yeast two‐hybrid interaction with residues 257–444 of FIP200 are shown with their number (#) and the observed interaction (+ or –).
-
B
Yeast two‐hybrid analysis for interaction between the above‐shown Rabaptin5 segments (Rbpt5#, fused to LexA on the bait plasmid) and residues 257–444 of FIP200 (FIP, fused to the Gal4 activation domain on the prey plasmid) to drive HIS3 expression. Three different clones each were replica‐plated on medium with His or without His, but containing 3‐amino‐1,2,4‐triazole (3AT; an inhibitor of His synthesis to increase stringency) and grown in the absence of Trp and leucine as a control. As negative controls, empty bait or prey plasmids were used. The asterisk indicates a clone invalidated by recombination.
-
C
Schematic representation of the sequence of FIP200 with its coiled‐coil segments in yellow. Residues 281–439 (gray) indicate the minimal sequence identified to interact with Rabaptin5 in the yeast two‐hybrid screen.
-
D
FIP200 was immunoprecipitated (IP) from lysates of HeLa or HEK293A cells and probed for FIP200, Rabaptin5 (Rbpt5), and EEA1 (early endosome antigen 1) by immunoblotting. Input lysate (10%) was immunoblotted blotted parallel. As a negative control, the immunoprecipitation was performed using an anti‐GAPDH antibody.
-
E–H
Lysates of HeLa cells transiently transfected with full‐length FIP200‐mCherry (FIP200‐mCh) or a deletion mutant lacking the segment interacting with Rabaptin5 (∆280–440) were immunoprecipitated with anti‐mCherry (IP FIP200‐mCh) or, as a control, with anti‐FLAG antibodies (IP FLAG). Immunoprecipitates and input lysates (10%) were immunoblotted for mCherry and Rabaptin5 (E), ATG13 (F), or ULK1 (G). Co‐immunoprecipitation of Rabaptin5, ATG13, and ULK1 with FIP200∆280–440 (FIP∆) was quantified in comparison with that with wild‐type FIP200 (H; signals normalized to that of the immunoprecipitated protein; mean ± SD of three independent experiments each).