Figure 2.
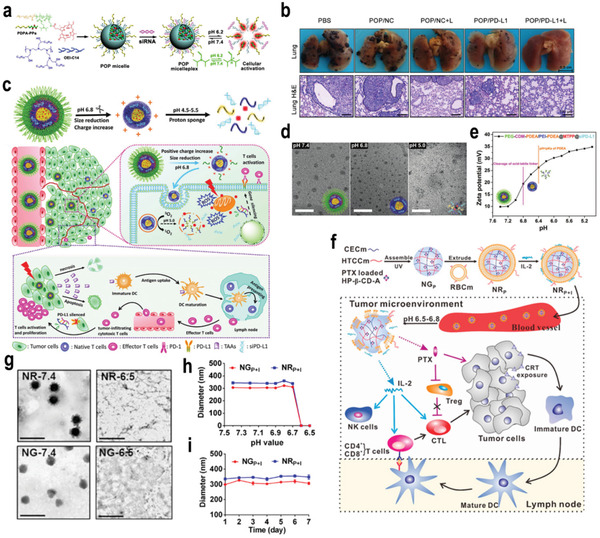
a) Chemical structure of the acid‐activatable POP micelleplexes coloaded with PPa and siRNA. (b) Photographs and H&E staining of the metastatic foci of the B16‐F10 tumors (scale bar 100 µm, n = 6). Reproduced with permission.[ 28 ] Copyright 2016, American Chemical Society. c) Illustration of pH‐response dissociable micelleplex‐mediated photodynamic tumor immunotherapy in vivo. d) TEM images of PCPP@MTPP@siPD‐L1 micelleplexes after various treatments with pH 7.4, pH 6.8, and pH 5.0 for 4 h. MTPP: (5‐(3‐Hydroxy‐p‐(4‐trimethylammonium) butoxyphenyl)‐10, 15, 20 triphenylporphyrin chlorine. e) Zeta potentials variation of PCPP@MTPP@siPD‐L1 micelleplexes under various pH value conditions. Reproduced with permission.[ 29 ] Copyright 2018, Wiley‐VCH. f) Preparation of NRP+I and schematic illustration of chemo‐immunotherapy. g) TEM images of NGP+I in pH 7.4, NGP+I in pH 6.5, NRP+I in pH 7.4, and NRP+I in pH 6.5. The scale bar is 1 µm. h) pH‐dependent particle sizes of NGP+I and NRP+I. i) In vitro stability of NGP+I and NRP+I in saline at 37 °C for 1 week. Reproduced with permission.[ 30 ] Copyright 2017, American Chemical Society. p‐Values: *p < 0.05; **p < 0.01; ***p < 0.001.
