Figure 2.
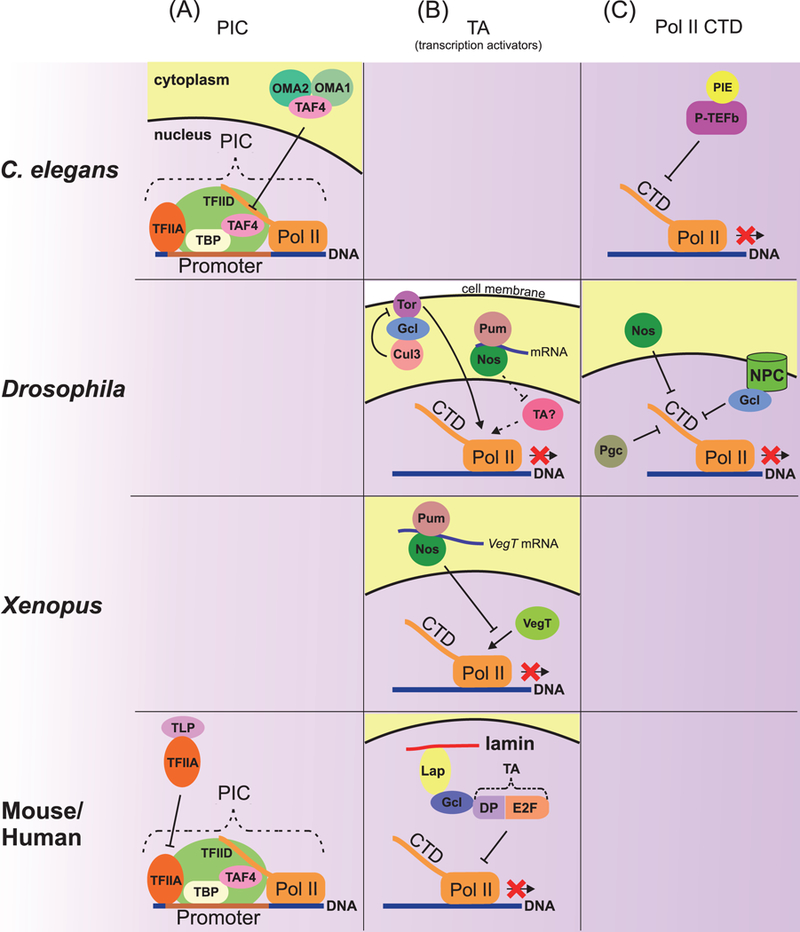
Mechanisms of repression of Pol II-dependent transcription. (A) Repression of transcription at the PIC assembly level. In C. elegans, germ plasm proteins OMA1 and 2 bind and retain the PIC component TAF4 in the cytoplasm, thus affecting PIC assembly in the nucleus. In mouse and human, the ТВР-related factor TLP binds with the unprocessed form of the general transcription factor TFIIA, thereby preventing completion of PIC assembly. (B) Repression of transcriptional activators. In Drosophila, Nos and Pum probably repress translation of an unknown transcriptional activator. In the Drosophila pole plasm, Gcl inactivates Torso signaling, which mediates somatic differentiation in terminal regions of the embryo. Gcl binds both Torso and the Cul3 ubiquitin ligase complex, which degrades Torso in the cell membrane. In Xenopus, Nos and Pum repress translation of the VegT transcriptional activator. In mouse and human, nuclear Gcl anchored to lamin through Lap physically interacts with the transcriptional factor DP-E2F and then inhibits its activity in the nuclear periphery. (C) Repression by a regulation of Pol II CTD phosphorylation. To start mRNA elongation, Pol II has to be modified by phosphorylation of Ser2 in CTD (shown in Fig. 1). In C. elegant, the germ plasm protein PIE inhibits P-TEFb kinase activity, which is required for phosphorylation of CTD Ser2. In Drosophila, the germ plasm proteins Nos, Gcl, and Pgc are involved in regulating CTD phosphorylation. Nuclear Gcl associated with NPC and cytoplasmic Nos inhibit phosphorylation of CTD by unknown mechanisms. Pgc affects CTD phosphorylation via inhibition of P-TEFb kinase activity (shown in detail in Fig. 4). Dashed lines indicate presumptive regulations.
