ABSTRACT
The orientation of epithelial cells in the plane of the tissue, known as planar cell polarity (PCP), is regulated by interactions of asymmetrically localized PCP protein complexes. In the Xenopus neural plate, Van Gogh-like2 (Vangl2) and Prickle3 (Pk3) proteins form a complex at the anterior cell boundaries, but how this complex is regulated in vivo remains largely unknown. Here, we use proximity biotinylation and crosslinking approaches to show that Vangl2–Pk3 association is inhibited by Frizzled3 (Fz3, also known as Fzd3), a core PCP protein that is specifically expressed in the neuroectoderm and is essential for the establishment of PCP in this tissue. This inhibition required Fz3-dependent Vangl2 phosphorylaton. Consistent with our observations, the complex of Pk3 with nonphosphorylatable Vangl2 did not polarize in the neural plate. These findings provide evidence for in vivo regulation of Vangl2–Pk3 complex formation and localization by a Frizzled receptor.
KEY WORDS: Vangl2, Phosphorylation, PCP, Neuroectoderm, Xenopus, Prickle3
Summary: Frizzled3 induces Vangl2 phosphorylation and promotes PCP in Xenopus neuroectoderm by inhibiting the formation of the anterior Vangl2–Prickle3 complex.
INTRODUCTION
Planar cell polarity (PCP) coordinates cell shape, orientation and cell movements that are necessary for normal morphogenesis (Butler and Wallingford, 2017; Devenport, 2014; Goodrich and Strutt, 2011). Initially discovered in Drosophila, ‘core PCP’ genes encode the transmembrane proteins Van Gogh (Vang), Frizzled (Fz), Flamingo/Starry Night (Fmi) and the cytosolic components Prickle (Pk) and Dishevelled (Dsh) (Adler, 2012; Peng and Axelrod, 2012). Besides controlling the orientation of epithelial cells, vertebrate PCP gene homologues acquired additional roles in the positioning and functions of subcellular structures, such as basal bodies and cilia, cell and tissue asymmetries, and diverse cell behaviors during development (Davey and Moens, 2017; Gray et al., 2011; Sokol, 2015).
A hallmark of PCP is the formation of the core PCP complexes Fmi–Fz–Dsh and Fmi–Vang–Pk that segregate to different cell sides (Peng and Axelrod, 2012). The partitioning of PCP complexes to opposite cell sides is based on their stabilization by intercellular and intracellular feedback interactions (Chen et al., 2008; Strutt et al., 2011; Tree et al., 2002; Wu and Mlodzik, 2008). Vang and Van Gogh-like 2 (Vangl2) can directly associate with Pk in vitro (Bastock et al., 2003; Chu et al., 2016; Jenny et al., 2003), but how this complex is regulated in developing vertebrate embryos remains unknown. Wnt5a- and Ror2-dependent phosphorylation of Vangl2 has been proposed to regulate PCP in the developing mouse limb (Gao et al., 2011). In Drosophila, Vang is also phosphorylated at the conserved sites in response to Fz (Kelly et al., 2016), but the mechanistic role of this phosphorylation for PCP signaling is not fully understood.
In the Xenopus neural plate, Vangl2 and Prickle3 (Pk3) form protein complexes that are enriched at the anterior side of each cell (Ossipova et al., 2015). Importantly, loss-of-function experiments have shown essential roles of Vangl2 in neural tube closure (Darken et al., 2002; Goto and Keller, 2002; Greene et al., 1998; Jessen et al., 2002; Kibar et al., 2001; Murdoch et al., 2001; Park and Moon, 2002). Frizzled3 (Fz3, also known as Fzd3) is another core PCP protein that is necessary for neural tube closure in mice (Wang et al., 2006). In this study, we use proximity biotinylation and crosslinking approaches (Choi-Rhee et al., 2004; Mattson et al., 1993; Roux et al., 2012) to demonstrate that Fz3 functions to establish neuroectodermal PCP and negatively regulates the association of Vangl2 and Pk3 in a manner that depends on Vangl2 phosphorylation in Xenopus embryos. Taken together, our findings reveal the regulation of the Vangl2–Pk3 complex by Fz3-dependent phosphorylation in vivo.
RESULTS
Frizzled3 is required for neural plate PCP
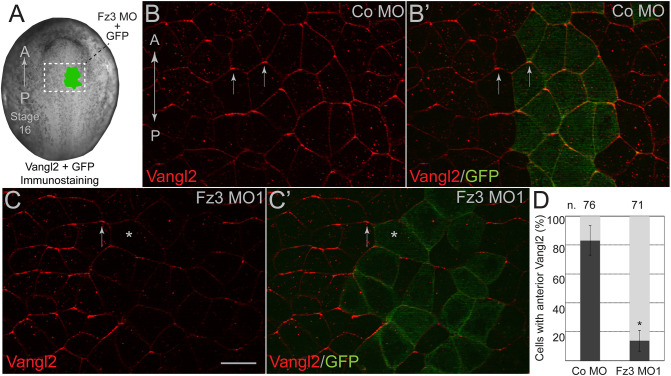
To evaluate potential regulation of the Vangl2–Pk3 complex by Frizzled, we studied Fz3, which is expressed in the Xenopus neural plate (Shi et al., 1998) and has been implicated in mouse neural tube closure (Wang et al., 2006). We first assessed the effect of Fz3 depletion on the localization of Vangl2, which has been shown to accumulate at anterior cell boundaries (Ossipova et al., 2015). Endogenous Vangl2 distribution was assessed by immunostaining of neurula-stage embryos injected with a previously characterized Fz3 morpholino oligonucleotide (Fz3 MO1) (Deardorff et al., 2001) together with GFP as a lineage tracer (Fig. 1A). The anterior enrichment of Vangl2 has been lost in the cells injected with Fz3 MO1 but not in those injected with control MO (Fig. 1B–D). However, Vangl2 was still present at the mediolateral borders of Fz3-depleted cells (Fig. S1A,B), suggesting that Vangl2 membrane localization is not affected. Notably, Fz3 morphants exhibited neural tube closure defects (Fig. S2A,B), consistent with the reported role of mouse Fz3 in neurulation (Wang et al., 2006). These experiments indicate that Fz3 is essential for the establishment of PCP in the Xenopus neural plate.
Fig. 1.
Frizzled3 is required for neural plate planar polarity. (A) Experimental scheme. One dorsal animal blastomere of a 16-cell Xenopus embryo was co-injected with Fz3 MO1 or control (Co) MO (5 ng each) and GFP RNA (200 pg). Stage 16 embryos were fixed and the neural plates were dissected and stained with anti-Vangl2 and anti-GFP antibodies. (B–C′) Representative en face images of neural plates from the injected embryos. Vangl2 (red) is enriched at the anterior cell edges (arrows). GFP is a lineage tracer (green). A-P, anterior-posterior axis. Scale bar: 30 µm. (B,B′) Co MO, (C,C′) Fz3 MO1. Many GFP-positive cells lack Vangl2 polarization (asterisk). (D) Quantification of the mean±s.d. frequencies for cells with anteriorly enriched Vangl2. Numbers of scored cells (n) are shown on top of each bar. Three embryos were scored for each group (20–30 cells per embryo). Data represent three different experiments. *P<0.05 (two-tailed unpaired Student's t-test).
Inhibition of the Vangl2–Pk3 interaction by Fz3
Since standard immunoprecipitation failed to detect the Vangl2–Pk3 complex in embryos (see below), this protein interaction was monitored by a proximity biotinylation assay. In this approach, we analyzed the biotinylation of exogenous Vangl2 by an N-terminal fragment of the biotin ligase from Aquifex aeolicus (Kim et al., 2016) that has been fused to Pk3 (BLN–Pk3) (Chuykin et al., 2018; Chuykin and Sokol, 2022; Reis et al., 2021). Co-expressed Fz3 inhibited Vangl2 biotinylation in a dose-dependent manner, indicating a negative regulation of the Vangl2–Pk3 complex (Fig. 2A). Notably, in the presence of Fz3, Vangl2 protein gel mobility has been reduced, whereas co-expression of Pk3 led to the faster migration of Vangl2 (Fig. 2A,B). These changes in Vangl2 mobility likely indicate phosphorylation, as previously demonstrated for Drosophila Vang (Kelly et al., 2016; Strutt et al., 2019).
Fig. 2.
Fz3 reduces the interaction between Vangl2 and Pk3 proteins in Xenopus embryos. (A) Vangl2 biotinylation by BLN–Pk3 is decreased in the presence of Fz3. Biotin and mRNAs encoding FLAG–BLN–Pk3 (400 pg), HA–Vangl2 or ΔPBD RNAs (100 pg each) and Fz3–FLAG (100 or 500 pg) as indicated, were injected into the animal region of 4- to 8-cell embryos. Protein lysates were immunoprecipitated (IP) with anti-HA antibody from stage 13 Xenopus embryos. Proteins were detected by immunoblotting (IB) with anti-biotin, anti-HA and anti-FLAG antibodies as indicated. Black arrowheads point to Vangl2 and Pk3, white arrowhead points to the slower migrating Vangl2 in pulldowns and lysates. Asterisks indicate endogenous proteins labeled by the anti-biotin antibody. (B) Lysates of embryos overexpressing Pk3 and Fz3 show that Pk3 increases Vangl2 mobility, whereas Fz3 reduces it. (C) Physical interaction of HA–Vangl2 and FLAG–Pk3 in transfected HEK293T cells. HA–Vangl2, but not the construct lacking the presumed Pk3-binding domain (ΔPBD, residues 298–382), is detected in pulldowns of FLAG–Pk3 from lysates of the transfected HEK293T cells. (D) HA-tagged Vangl2 but not ΔPBD is biotinylated by FLAG–BLN–Pk3. Embryo microinjection details and abbreviations are as in A. (E) Model. The formation of a complex between Vangl2 and Pk3 results in the biotinylation of Vangl2 by BLN–Pk3, green circles depict biotin. Fz3 causes an upshift of Vangl2 due to phosphorylation (circled red P) and inhibits the Vangl2–Pk3 interaction. Data is representative of three experiments.
We wanted to confirm that Vangl2 biotinylation truly reflects the physical proximity of the two proteins and generated a Vangl2 deletion construct lacking the presumed Pk3-binding domain (PBD) of Vangl2 (Jenny et al., 2003). As we expected, the Vangl2ΔPBD construct failed to bind Pk3 in transfected HEK293T cells (Fig. 2C) and has not been biotinylated by BLN–Pk3 (Fig. 2D), thereby verifying our approach. These observations indicate that Fz3 inhibits the association of Vangl2 and Pk3 in Xenopus embryos (Fig. 2E).
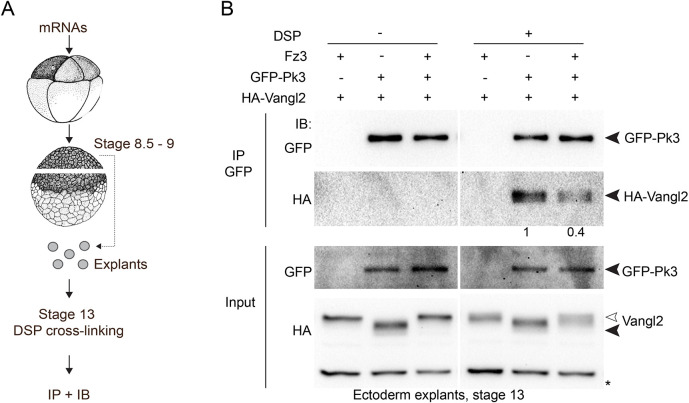
To further verify our conclusion regarding the negative role of Fz3 in the formation of the Vangl2–Pk3 complex, we used immunoprecipitation combined with crosslinking. Whereas the Vangl2–Pk3 complex is readily detectable by immunoprecipitation in cultured cells (Fig. 2C) (Chu et al., 2016), our initial attempts to visualize it in embryos were unsuccessful. We suspected that the Vangl2–Pk3 complex is unstable in a detergent-containing buffer and stabilized it by crosslinking with dithiobis-succinimidyl-propionate (DSP, see Materials and Methods) (Fig. 3A). Indeed, Vangl2 was present in Pk3 pulldowns prepared from the crosslinked ectodermal explants, but not in the samples without crosslinking (Fig. 3B). Importantly, lower amounts of Vangl2 coprecipitated with Pk3 from Fz3-expressing explants (Fig. 3B). Notably, Pk3 preferentially associated with the non-phosphorylated (fast migrating) form of Vangl2. By contrast, both phosphorylated and non-phosphorylated forms of Vangl2 were visible in the lysates from cells expressing Fz3 (Fig. 3B). These observations suggest that the PCP complex is modulated by Vangl2 phosphorylation. Taken together, our results validate proximity biotinylation for the analysis of Vangl2–Pk3 association in vivo and reveal the negative regulation of this complex by Fz3.
Fig. 3.
Fz3 decreases the amount of the Vangl2–Pk3 complex in vivo. (A) Experimental scheme; 8-cell embryos were injected into each animal blastomere with GFP–Pk3 (200 pg), HA–Vangl2 (50 pg) or Fz3–FLAG (400 pg) mRNA as indicated. Ectoderm explants were dissected at stage 8.5–9, cultured until stage 13, and cross-linked with 2 mM DSP for 30 min. After immunoprecipitation (IP) with GFP-trap beads, protein levels were assessed by immunoblotting (IB) with anti-HA and anti-GFP antibodies. (B) Vangl2 and GFP–Pk3 co-precipitation in control and Fz3-expressing ectoderm explants (right). No binding is detected without cross-linking (left). Black arrowheads point to Vangl2 and Pk3, white arrowhead points to the slower migrating Vangl2 band in the lysates. A nonspecific band reflects protein loading (asterisk). The numbers are band intensity ratios for HA–Vangl2 and GFP–Pk3 in precipitated complexes. Data are representative of three experiments.
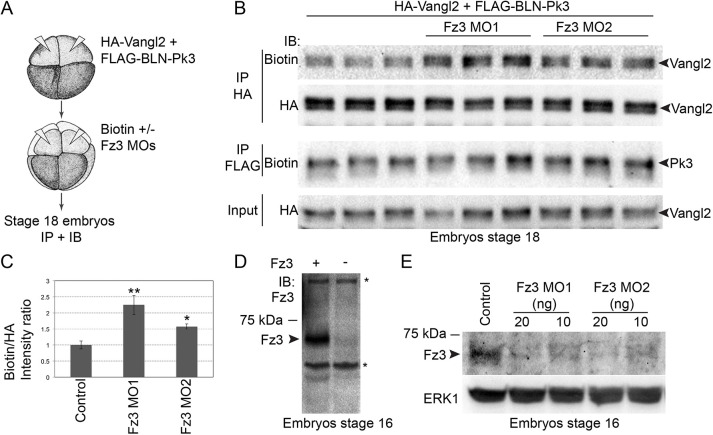
We next assessed the effect of Fz3 depletion on Vangl2–Pk3 complex formation. Given the dramatic increase in Fz3 transcription during neurulation (Deardorff et al., 2001; Shi et al., 1998), we measured Vangl2 biotinylation at stage 18 to allow sufficient time for protein depletion (Fig. 4A). Fz3 depletion with two different MOs consistently increased Vangl2 biotinylation by BLN-Pk3 (Fig. 4B,C). Lack of detectable changes in Vangl2 mobility in Fz3 morphants is likely to be due to limited assay sensitivity. We also found that the levels of endogenous Fz3 were reduced in stage 16–17 morphants, confirming the efficiency of Fz3 MOs (Fig. 4D,E). These results further support our conclusion that Fz3 inhibits the interaction between Vangl2 and Pk3 in vivo.
Fig. 4.
The interaction between Vangl2 and Prickle3 is enhanced in Fz3-depleted embryos. (A) Experimental scheme. 4- to 8-cell embryos were sequentially injected into dorsal animal blastomeres with BLN–Pk3 and HA–Vangl2 DNAs (50 pg each), and Fz3 MO1 or Fz3 MO2 with biotin. Embryo lysates were collected at stage 18 for immunoprecipitation (IP) and immunoblotting (IB). (B) Vangl2 and Pk3 biotinylation in control and Fz3 morphant embryos in pulldowns were assessed with anti-biotin antibodies. Total exogenous Vangl2 levels were analyzed by anti-HA antibodies. Owing to low abundance and insufficient sensitivity of the detection, the levels and the activity of FLAG–BLN–Pk3 were assessed in the second sequential FLAG pulldown. Biological triplicates were performed for each experimental condition with 20 embryos per sample. (C) Band intensity ratios of biotinylated to total Vangl2 levels in pulldowns, evaluated with anti-biotin and anti-HA antibodies, respectively. Results are mean±s.d. (n=3). *P<0.05; **P<0.001 (two-tailed unpaired Student's t-test). (D) Anti-Fz3 antibody detects Fz3 (arrowhead) in lysates of stage 16 control embryos or embryos injected with Fz3-FLAG mRNA (25 pg); asterisks indicate non-specific bands. (E) Levels of the endogenous Fz3 protein (arrowhead) in control, Fz3 MO1- and Fz3 MO2-injected stage 16 embryos; ERK1 is a loading control. Data in D,E are representative of three experiments.
Fz3 promotes phosphorylation of Vangl2 at threonine sites
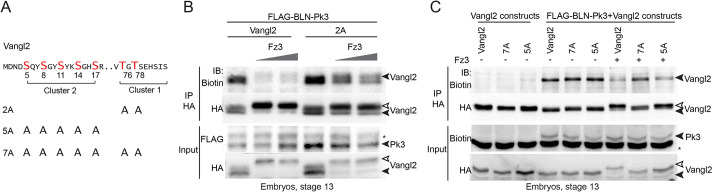
Mouse Vangl2 can be phosphorylated at multiple conserved serine/threonine (S/T) residues that belong to two N-terminal clusters (Gao et al., 2011). To identify specific residues that are phosphorylated in response to Fz3, we generated several mutated Xenopus Vangl2 constructs (Fig. 5A), expressed them in the embryo and probed lysates with antibody specific to Vangl2 phosphorylated at S82 and S84 (Gao et al., 2011) and anti-phospho-threonine antibodies. Phosphopeptide antibody specificity was confirmed by lack of staining of the sample containing S82S84>AA (Fig. 5B). The phosphorylation of the S82 and S84 sites did not change in Fz3-expressing ectoderm. By contrast, threonine phosphorylation was induced in several Vangl2 constructs after Fz3 overexpression (Fig. 5B). No change in threonine phosphorylation was observed in the S82S84>AA construct, consistent with S82 and S84 being the founder sites that are necessary for subsequent phosphorylation of other cluster I residues (Gao et al., 2011). Importantly, no increase in phospho-threonine levels was observed in the T76T78>AA construct (Fig. 5C). We therefore propose that T76 and T78 are phosphorylated in response to Fz3 signaling.
Fig. 5.
Phosphorylation of Vangl2 at threonine residues is increased in Fz3-expressing embryos. (A) Scheme of Vangl2 constructs with the modified cytoplasmic N-terminal region (amino acids 1–84, blue) used to analyze Vangl2 phosphorylation. Phosphorylated (p-)serine and threonine (S/T) residues in clusters I and II are indicated. (B,C) Immunoprecipitations (IP) with anti-HA antibodies from lysates of embryos expressing Vangl2 constructs with or without Fz3 were immunoblotted (IB) with phospho-specific antibodies as indicated. Anti-HA antibodies show total Vangl2 levels. Asterisk in B marks non-specific band recognized by anti-HA antibody in lysates. (C) Phosphothreonine phosphorylation in Vangl2 requires T76 and T78. A Vangl2 upshift is visible for the T76T78>AA mutant (white arrowheads). Data are representative of three experiments.
Putative Vangl2 phosphorylation at T76 and T78 is required for the inhibitory activity of Fz3 on Vangl2–Pk3 association and planar polarity
The observed correlation between the reduced Vangl2–Pk3 interaction and the increase in the slow migrating Vangl2 band in Fz3-expressing embryos (Fig. 2B) suggests that Fz3 inhibits Vangl2–Pk3 complex formation by triggering Vangl2 phosphorylation. To address this hypothesis, we tested several Vangl2 constructs with alanine substitutions for the conserved serine and threonine residues (Fig. 6A). We found that the interaction of Pk3 with the T76T78>AA construct was barely inhibited by Fz3 (Fig. 6B). Consistently, GFP–Pk3 cortical localization was inhibited by Fz3 in the presence of Vangl2 (Fig. S3). The effect of Fz3 was partial in the presence of T76T78>AA (Fig. S3). Furthermore, the T76T78>AA mutant caused pronounced neural tube defects as compared to Vangl2 (Fig. S4). Because the effect of T76T78>AA on Fz3 activity was incomplete, we thought that the serine residues of cluster II might contribute to the modulation of the PCP complex. This possibility was confirmed after testing additional Vangl2 alanine substitutions. The association of Pk3 with Vangl2-7A was resistant to Fz3, whereas the complex containing Vangl2-5A was partly sensitive (Fig. 6A,C). These experiments indicate the contributing role of cluster II serine residues for the inhibitory effect of Fz3 on PCP complex formation.
Fig. 6.
Fz3-induced Vangl2 phosphorylation is necessary for the inhibition of the Vangl2–Pk3 interaction. (A) Scheme of Vangl2 constructs with alanine substitutions. Conserved serine and threonine (S/T) clusters I and II are indicated. (B,C) Effect of Fz3 on the biotinylation of various Vangl2 constructs by BLN–Pk3. Embryos were injected dorsoanimally with mRNAs encoding Vangl2 constructs (50 pg each), BLN–Pk3 (200 pg), or Fz3–FLAG mRNA (200 pg or 400 pg) (B) and 400 pg (C); and collected at stage 13. (B) The Vangl2-2A mutant is partly resistant to the Fz3 inhibitory effect. (C) Vangl2-7A is completely resistant to Fz3 and is not upshifted (black and white arrowheads). Immunoblotting (IB) with anti-biotin, anti-HA and anti-FLAG antibodies was done as indicated. Asterisks represent a non-specific band. Data are representative of three experiments.
We next assessed the contribution of Vangl2 phosphorylation to PCP. Vangl2 was compared with Vangl2-7A, which does not dissociate from the Pk3 protein in response to Fz3. Polarized complexes of Pk3 and Vangl2 were readily detectable at anterior cell faces in the neural plate at stage 14, whereas the complexes of Pk3 and Vangl2-7A were more randomly distributed along the cell cortex (Fig. 7A–C). Taken together, our data are consistent with a model in which Vangl2 phosphorylation by Fz3 is necessary for the anterior enrichment of the Vangl2–Pk3 complex (Fig. 7D).
Fig. 7.
Fz3-induced Vangl2 phosphorylation is required for PCP. (A–C) Two dorsal blastomeres of 16-cell embryos were co-injected with HA–Vangl2 or HA–Vangl2-7A RNA (50 pg) and GFP–Pk3 RNA (200 pg). (A–B′) Representative fixed stage 14 neural plates have been imaged for GFP–Pk3 (direct GFP fluorescence) and immunostained for HA–Vangl2 (A,A′) or HA–Vangl2-7A (B,B′). (A,A′) Polarized anterior PCP complexes are indicated by arrows. (B,B′) Cells lacking anterior GFP–Pk3 are marked by asterisks. Scale bar: 30 µm. Anteroposterior (AP) axis is indicated. (C) Quantification of GFP–Pk3 fluorescence for PCP complexes containing wild-type Vangl2 (blue) and those containing Vangl2-7A (orange) in mosaically expressing cells. Mean±s.d. is shown along the cell circumference for multiple cells as a function of the circular angle from 0 to 360 degrees relative to the AP axis (as shown on the scheme). Numbers of scored cells per each group are indicated. Data are representative of three independent experiments. (D) Model. Pk3 binding to Vangl2 promotes the formation of the anterior PCP complex in neuroectoderm cells. The anterior accumulation of the complex is reinforced by Fz3 that triggers T76T78 phosphorylation of Vangl2 and inhibits the Vangl2–Pk3 interaction. Presumptive posterior Fz3 localization is shown by the dashed line.
DISCUSSION
Our experiments reveal a role of Fz3 in the inhibition of Vangl2–Pk3 association in the Xenopus neural plate. This result supports the hypothesis that negative regulatory feedback between core PCP complexes leads to their segregation to opposite cell sides. In Drosophila, Pk has been shown to negatively impact Fz activity (Tree et al., 2002). In vertebrates, Vangl2 can affect the phosphorylation and trafficking of Fz3, although the relevance of this observation to PCP remains to be confirmed (Shafer et al., 2011). Although the interaction of Vangl2 and Prickle is commonly assumed to be constitutive, we show its modulation by Fz3. We hypothesize that Fz3 prevents the formation of the Vangl2–Pk3 complex at the posterior side of each cell by inducing Vangl2 phosphorylation at T76 and T78 residues (Fig. 7D). Based on our analysis of nonphosphorylatable Vangl2 constructs, we propose that Vangl2 phosphorylation is required for the establishment of PCP in the neural plate.
If Fz3 antagonizes the Vangl2–Pk3 complex in a cell-autonomous manner, it is expected to localize in a pattern that is complementary to the distribution of the Vangl2–Pk3 aggregates. So far, the presence of the Fz3-containing complex at the posterior sides of neuroepithelial cells has not been documented. Nevertheless, asymmetric localization of Frizzled proteins has been reported in other vertebrate tissues, including tracheal and kidney epithelia, inner ear and brain ventricles (Davey et al., 2016; Kunimoto et al., 2017; Montcouquiol et al., 2006; Vladar et al., 2012), consistent with a conserved signaling mechanism.
We hypothesize that T76 and T78 are the specific amino acid residues that are phosphorylated in response to Fz3 and demonstrate their requirement for the inhibition of Vangl2-Pk3 association. The substitution of alanine residues for the threonine residues at these sites together with the additional substitutions of previously identified serine residues (Gao et al., 2011) disrupts the anterior enrichment of Vangl2–Pk3 complex, supporting the idea that Vangl2 phosphorylation has a role in PCP. Although Fz3 stimulates Vangl2 phosphorylation, we observed no changes in Vangl2 protein mobility in Fz3-depleted embryos. This negative result might be due to the limited sensitivity of the gel shift assay. Although CK1δ and CK1ε kinases have been implicated in the phosphorylation of the conserved S82/S84 sites in mouse Vangl2 (Gao et al., 2011; Yang et al., 2017) and the corresponding sites in Drosophila Vang (Kelly et al., 2016; Strutt et al., 2019), further studies are needed to identify the protein kinase that is activated by Fz3 and phosphorylates the T76/T78 sites.
How does Fz3 affect the interaction of Vangl2 and Pk3? One possibility is that the phosphorylation prevents Vangl2 oligomerization and clustering that could be affecting the interaction with Prickle (Belotti et al., 2012; Yin et al., 2012). Alternatively, Fz3 activity may indirectly modulate Vangl2 by affecting another PCP component, such as Ror2 (Gao et al., 2011), Celsr/Fmi (Chen et al., 2008), Dvl (Bastock et al., 2003; Yang et al., 2017) or Par3 (Chuykin et al., 2018).
Although the requirement of PCP molecules in neural tube closure has been established in genetic studies (Nikolopoulou et al., 2017), how they operate during neurulation is still unclear. PCP complexes have been hypothesized to trigger actomyosin contractility and cell junction shrinking (McGreevy et al., 2015; Nishimura et al., 2012), but the direct data are still missing. One possibility is that core PCP proteins interact with and modulate actin-remodeling proteins, as has been proposed for the actin-binding protein Shroom3 (Hildebrand and Soriano, 1999; McGreevy et al., 2015; Ossipova et al., 2014). Conversely, PCP proteins may themselves be regulated by actomyosin-dependent forces (Chien et al., 2015; Mahaffey et al., 2013; Newman-Smith et al., 2015; Ossipova et al., 2015). These mechanisms are consistent with the genetic evidence that Rock/Myosin II signaling is required for fly PCP (Strutt et al., 1997; Winter et al., 2001). Notably, the amphibian neural tube closure proceeds in the posterior-to-anterior direction, correlating with the more pronounced Vangl2 polarity in the posterior neural plate (Mancini et al., 2021; Ossipova et al., 2015). Future studies should address the possible connection between the Fz3-dependent Vangl2 phosphorylation that we described and cell movements during neurulation.
MATERIALS AND METHODS
Plasmids, mRNA synthesis and morpholino oligonucleotides
Plasmids encoding Xenopus GFP-, FLAG- and FLAG-BLN-tagged Pk3, HA-Vangl2 and Fz3-FLAG were described previously (Chu et al., 2016; Chuykin et al., 2018; Shi et al., 1998). HA-tagged deletion mutants of Vangl2 Δ1-28, Δ29-62, Δ1-62, Δ298-382 and Vangl2 S/T>A mutations were generated by site-directed mutagenesis or sub-cloning of gBlock fragments (Integrated DNA Technologies) in pCS2 (Turner and Weintraub, 1994). Capped mRNAs were synthesized using mMessage mMachine kit (Ambion, Austin, TX). For depletion studies, the following MOs were purchased from Gene Tools (Philomath, OR): Fz3 MO1, 5′-CGCAAAGCCACATGCACCTCTTGAA-3′ (Deardorff et al., 2001); Fz3 MO2, 5′-CTTGCGTCCAAAGCACCAATTGCTC-3′; and control MO (Co MO), 5′-AGCGTTTCAGGCCGATCTCTCAGTC-3′.
Xenopus embryo culture and microinjections
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal protocol was approved by the IACUC of the Icahn School of Medicine at Mount Sinai. Eggs were obtained from Xenopus laevis females, in vitro fertilized and cultured in 0.1× Marc's modified Ringer's solution (MMR) as described previously (Newport and Kirschner, 1982). Staging was according to Nieuwkoop and Faber (1967). For microinjections, 4–16-cell embryos were transferred to 3% Ficoll (GE Healthcare) in 0.6× MMR and injected with 5–10 nl of a solution containing mRNAs and/or MOs. For mosaic expression of PCP complexes, embryos were injected into two dorsal blastomeres of 16-cell embryos. Amounts of injected mRNAs have been optimized in the preliminary dose–response experiments and are indicated in figure legends.
Cell culture, transfection and immunoprecipitation
HEK293T cells (ATCC) were maintained in Dulbecco's modified Eagles medium (Corning) with 10% fetal bovine serum (Sigma) and penicillin/streptomycin (Sigma). Cells growing at 50–70% confluence were transiently transfected using linear polyethylenimine (MW 25,000, Polysciences) as described previously (Chuykin et al., 2018). For transfections, each 60-mm well received 1 µg of plasmids encoding FLAG–Pk3, HA–Vangl2 WT or HA–Vangl2Δ298-382 constructs in pCS2. Vector DNA (pCS2) was added to plasmid DNA mixture to reach the total amount of 3 µg. For immunoprecipitation, cells were harvested 2 days after transfections in the buffer containing 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA and 1% Triton X-100. Lysates were incubated for several hours or overnight with 5 µl of M2 agarose beads (Sigma) at 4°C on a rotating shaker. The beads were washed twice with the lysis buffer, and the samples were heated at 95°C in the sample buffer for separation in SDS-PAGE.
Immunostaining, fluorescent protein detection, imaging and quantification
To detect PCP complexes in the neural plate, embryos were collected at stage 14 and the vitelline membrane was removed manually. GFP–Pk3 mRNA was co-expressed with Vangl2 mRNA at the previously established doses (as indicated in the figure legends), which have no effect on normal development. Embryos were fixed in MEMFA solution (100 mM MOPS pH 7.4, 2 mM EGTA, 1 mM MgSO4, 3.7 % formaldehyde) for 40 min, washed in 0.3% Triton X-100 in PBS, and neural plate explants were dissected, immunostained with anti-HA antibody (1:100, 12CA5 hybridoma supernatant) to detect HA–Vangl2, imaged and scored. For detection of endogenous Vangl2, embryos were collected at stage 16, fixed in 2% trichloroacetic acid (TCA) for 30 min, and the immunostaining was performed with the rabbit polyclonal anti-Vangl2 antibody (1:100; Ossipova et al., 2015). For the tracing of Fz3-depleted cells, embryos were co-injected with MOs and GFP mRNA, and co-immunostained with anti-Vangl2 and with mouse monoclonal anti-GFP antibody (1:100; GFP-B2, Santa Cruz Biotechnology). Secondary antibodies were against mouse or rabbit IgG conjugated to Cy2 or Cy3 (1:500, Jackson ImmunoResearch). GFP–Pk3 was detected by epifluorescence. Explants were mounted in the Vectashield mounting medium (Vector). Standard specificity controls were performed to confirm the lack of cross-reactivity and no staining without primary antibodies.
Images that are representative of at least 10 different fields were captured using a Zeiss AxioImager microscope with the Apotome attachment. The data shown are from two to five independent experiments with 5–15 embryos per group. To quantify the effect of Fz3 depletion on PCP, cells with anteriorly enriched Vangl2 were scored for three embryos per group, and 20–30 cells per embryo, after the embryos have been injected with Fz3 MO or Co MO with GFP as a tracer. Statistical significance was determined by the two-tailed unpaired Student's t-test (Excel). Quantification of fluorescence intensity of endogenous Vangl2 at lateral cell boundaries was undertaken with the Fiji software. The quantification of planar polarity of the of (GFP–Pk3)–(HA–Vangl2) complexes (Vangl2 versus Vangl2-7A constructs) was performed using the Fiji software with the Azimuthal Average plugin https://imagej.nih.gov/ij/plugins/azimuthal-average.html. Mosaic cells were selected using the polygon tool in Fiji and GFP-Pk3 fluorescence intensity was quantified along the radius in cells expressing PCP complexes, the calculated means were plotted as a graph (Microsoft Excel). Data represent three independent experiments and were verified blindly.
Proximity biotinylation in Xenopus embryos and immunoblotting
For proximity biotinylation, embryos were injected into the animal pole of 4–8-cell embryos with 10 nl of the solution containing 0.8 mM of biotin and mRNAs encoding FLAG–BLN-Pk3 (200 or 400 pg), HA–Vangl2, (50 pg) and Fz3–FLAG (200 to 700 pg). Embryos were lysed and the protein biotinylation was assessed in embryo lysates and pulldowns with mouse anti-HA (10 μl of hybridoma supernatant; 12A5) and anti-FLAG (4 μl of FLAG agarose; M2) antibodies. Stage is indicated in the figure legends. Proteins were detected by the immunoblotting with goat anti-biotin-HRP antibodies (1:3000; cat. no. 7075, Cell Signaling) or goat anti-biotin antibody (1:30,000; cat. no. 31852, Pierce), anti-FLAG (1:1000; cat. no. F3165, M2 Sigma), rabbit anti-phosphothreonine (1:8000; cat. no. 1607, EMD Millipore), rabbit anti-HA (1:5000; cat. no. A190-108A, Bethyl), anti-ERK1 (1:2000; cat. no. sc94, Santa Cruz Biotechnology) and mouse monoclonal anti-Xenopus Fz3 antibodies (a gift of Peter Klein, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA; Deardorff et al., 2001).
Analysis of the Vangl2–Pk3 binding in crosslinked ectoderm explants
Four- to eight-cell embryos were injected into all four animal blastomeres with mRNAs encoding GFP–Pk3 (200 pg) and HA–Vangl2 (50 pg) with or without Fz3–FLAG (400 pg). Ectoderm explants were isolated at stage 8.5–9 and cultured until stage 13. A total of 30 explants were collected in 50 µl of 0.6× MMR solution and treated with 2 mM dithiobis-succinimidyl-propionate (DSP) (Thermo Fisher Scientific) (Mattson et al., 1993) for 30 min, and were then lysed. Pulldown of GFP-Pk3 was performed with GFP-Trap (Chromotek) followed by immunoblotting.
Supplementary Material
Acknowledgement
We thank Peter Klein and De-Li Shi for Xenopus Fz3 plasmids and monoclonal antibodies specific for Fz3, Chih-Wen Chu for Fz3 MO2, Yingzi Yang for pS82/S84 phosphopeptide-specific antibodies and Shilpa Dilip Kumar for advice on quantification. We also thank Miho Matsuda for comments on the manuscript and members of the Sokol laboratory for discussions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: I.C., S.S.; Methodology: I.C., K.I., K.K., S.S.; Validation: I.C., K.I., K.K.; Formal analysis: I.C., K.I., S.S.; Investigation: I.C., K.I., K.K.; Resources: S.S.; Writing - original draft: I.C., S.S.; Writing - review & editing: I.C., K.I., S.S.; Visualization: I.C., K.K.; Supervision: S.S.; Funding acquisition: S.S.
Funding
This study has been supported by National Institutes of Health grants GM122492 and NS100759 to S.Y.S. Deposited in PMC for release after 12 months.
Peer review history
The peer review history is available online at https://journals.biologists.com/jcs/article-lookup/doi/10.1242/jcs.258864.
References
- Adler, P. N. (2012). The frizzled/stan pathway and planar cell polarity in the Drosophila wing. Curr. Top. Dev. Biol. 101, 1-31. 10.1016/B978-0-12-394592-1.00001-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock, R., Strutt, H. and Strutt, D. (2003). Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development 130, 3007-3014. 10.1242/dev.00526 [DOI] [PubMed] [Google Scholar]
- Belotti, E., Puvirajesinghe, T. M., Audebert, S., Baudelet, E., Camoin, L., Pierres, M., Lasvaux, L., Ferracci, G., Montcouquiol, M. and Borg, J. P. (2012). Molecular characterisation of endogenous Vangl2/Vangl1 heteromeric protein complexes. PLoS ONE 7, e46213. 10.1371/journal.pone.0046213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, M. T. and Wallingford, J. B. (2017). Planar cell polarity in development and disease. Nature Publishing Group 18, 375-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W.-S., Antic, D., Matis, M., Logan, C. Y., Povelones, M., Anderson, G. A., Nusse, R. and Axelrod, J. D. (2008). Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling. Cell 133, 1093-1105. 10.1016/j.cell.2008.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien, Y. H., Keller, R., Kintner, C. and Shook, D. R. (2015). Mechanical strain determines the axis of planar polarity in ciliated epithelia. Curr. Biol. 25, 2774-2784. 10.1016/j.cub.2015.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi-Rhee, E., Schulman, H. and Cronan, J. E. (2004). Promiscuous protein biotinylation by Escherichia coli biotin protein ligase. Protein Sci. 13, 3043-3050. 10.1110/ps.04911804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, C.-W., Ossipova, O., Ioannou, A. and Sokol, S. Y. (2016). Prickle3 synergizes with Wtip to regulate basal body organization and cilia growth. Sci. Rep. 6, 24104. 10.1038/srep24104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuykin, I., Ossipova, O. and Sokol, S. Y. (2018). Par3 interacts with Prickle3 to generate apical PCP complexes in the vertebrate neural plate. Elife 7, e37881. 10.7554/eLife.37881.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuykin, I. and Sokol, S. Y. (2022) Analysis of planar cell polarity complexes by proximity biotinylation in Xenopus embryos. Methods Mol. Biol. 2438, in press. [DOI] [PubMed] [Google Scholar]
- Darken, R. S., Scola, A. M., Rakeman, A. S., Das, G., Mlodzik, M. and Wilson, P. A. (2002). The planar polarity gene strabismus regulates convergent extension movements in Xenopus. EMBO J. 21, 976-985. 10.1093/emboj/21.5.976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey, C. F. and Moens, C. B. (2017). Planar cell polarity in moving cells: think globally, act locally. Development 144, 187-200. 10.1242/dev.122804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey, C. F., Mathewson, A. W. and Moens, C. B. (2016). PCP Signaling between migrating neurons and their planar-polarized neuroepithelial environment controls filopodial dynamics and directional migration. PLoS Genet. 12, e1005934. 10.1371/journal.pgen.1005934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff, M. A., Tan, C., Saint-Jeannet, J. P. and Klein, P. S. (2001). A role for frizzled 3 in neural crest development. Development 128, 3655-3663. 10.1242/dev.128.19.3655 [DOI] [PubMed] [Google Scholar]
- Devenport, D. (2014). The cell biology of planar cell polarity. J. Cell Biol. 207, 171-179. 10.1083/jcb.201408039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, B., Song, H., Bishop, K., Elliot, G., Garrett, L., English, M. A., Andre, P., Robinson, J., Sood, R., Minami, Y.et al. (2011). Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev. Cell 20, 163-176. 10.1016/j.devcel.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich, L. V. and Strutt, D. (2011). Principles of planar polarity in animal development. Development 138, 1877-1892. 10.1242/dev.054080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, T. T. and Keller, R. R. (2002). The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev. Biol. 247, 17-17. 10.1006/dbio.2002.0673 [DOI] [PubMed] [Google Scholar]
- Gray, R. S., Roszko, I. and Solnica-Krezel, L. (2011). Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev. Cell 21, 120-133. 10.1016/j.devcel.2011.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene, N. D., Gerrelli, D., Van Straaten, H. W. and Copp, A. J. (1998). Abnormalities of floor plate, notochord and somite differentiation in the loop-tail (Lp) mouse: a model of severe neural tube defects. Mech. Dev. 73, 59-72. 10.1016/S0925-4773(98)00029-X [DOI] [PubMed] [Google Scholar]
- Hildebrand, J. D. and Soriano, P. (1999). Shroom, a PDZ domain-containing actin-binding protein, is required for neural tube morphogenesis in mice. Cell 99, 485-497. 10.1016/S0092-8674(00)81537-8 [DOI] [PubMed] [Google Scholar]
- Jenny, A., Darken, R. S., Wilson, P. A. and Mlodzik, M. (2003). Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. EMBO J. 22, 4409-4420. 10.1093/emboj/cdg424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen, J. R. J., Topczewski, J. J., Bingham, S. S., Sepich, D. S. D., Marlow, F. F., Chandrasekhar, A. A. and Solnica-Krezel, L. L. (2002). Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat. Cell Biol. 4, 610-615. 10.1038/ncb828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, L. K., Wu, J., Yanfeng, W. A. and Mlodzik, M. (2016). Frizzled-induced van gogh phosphorylation by CK1ε promotes asymmetric localization of core PCP factors in Drosophila. Cell Reports 16, 344-356. 10.1016/j.celrep.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibar, Z., Vogan, K. J., Groulx, N., Justice, M. J., Underhill, D. A. and Gros, P. (2001). Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat. Genet. 28, 251-255. 10.1038/90081 [DOI] [PubMed] [Google Scholar]
- Kim, D. I., Jensen, S. C., Noble, K. A., Kc, B., Roux, K. H., Motamedchaboki, K. and Roux, K. J. (2016). An improved smaller biotin ligase for BioID proximity labeling. Mol. Biol. Cell 27, 1188-1196. 10.1091/mbc.E15-12-0844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimoto, K., Bayly, R. D., Vladar, E. K., Vonderfecht, T., Gallagher, A. R. and Axelrod, J. D. (2017). Disruption of core planar cell polarity signaling regulates renal tubule morphogenesis but is not cystogenic. Curr. Biol. 27, 3120-3131.e4. 10.1016/j.cub.2017.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey, J. P., Grego-Bessa, J., Liem, K. F. and Anderson, K. V. (2013). Cofilin and Vangl2 cooperate in the initiation of planar cell polarity in the mouse embryo. Development 140, 1262-1271. 10.1242/dev.085316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini, P., Ossipova, O. and Sokol, S. Y. (2021). The dorsal blastopore lip is a source of signals inducing planar cell polarity in the Xenopus neural plate. Biol. Open 10, bio058761. 10.1242/bio.058761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson, G., Conklin, E., Desai, S., Nielander, G., Savage, M. D. and Morgensen, S. (1993). A practical approach to crosslinking. Mol. Biol. Rep. 17, 167-183. 10.1007/BF00986726 [DOI] [PubMed] [Google Scholar]
- McGreevy, E. M., Vijayraghavan, D., Davidson, L. A. and Hildebrand, J. D. (2015). Shroom3 functions downstream of planar cell polarity to regulate myosin II distribution and cellular organization during neural tube closure. Biol. OPEN 4, 186-196. 10.1242/bio.20149589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol, M., Sans, N., Huss, D., Kach, J., Dickman, J. D., Forge, A., Rachel, R. A., Copeland, N. G., Jenkins, N. A., Bogani, D.et al. (2006). Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J. Neurosci. 26, 5265-5275. 10.1523/JNEUROSCI.4680-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch, J. N., Doudney, K., Paternotte, C., Copp, A. J. and Stanier, P. (2001). Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum. Mol. Genet. 10, 2593-2601. 10.1093/hmg/10.22.2593 [DOI] [PubMed] [Google Scholar]
- Newman-Smith, E., Kourakis, M. J., Reeves, W., Veeman, M. and Smith, W. C. (2015). Reciprocal and dynamic polarization of planar cell polarity core components and myosin. Elife 4, e05361. 10.7554/eLife.05361.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport, J. and Kirschner, M. (1982). A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell 30, 675-686. 10.1016/0092-8674(82)90272-0 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop, P. D. and Faber, J. (1967). Normal Table of Xenopus laevis. Amsterdam: North-Holland Publishing Company. [Google Scholar]
- Nikolopoulou, E., Galea, G. L., Rolo, A., Greene, N. D. E. and Copp, A. J. (2017). Neural tube closure: cellular, molecular and biomechanical mechanisms. Development 144, 552-566. 10.1242/dev.145904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, T. T., Honda, H. H. and Takeichi, M. M. (2012). Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell 149, 1084-1097. 10.1016/j.cell.2012.04.021 [DOI] [PubMed] [Google Scholar]
- Ossipova, O., Kim, K., Lake, B. B., Itoh, K., Ioannou, A. and Sokol, S. Y. (2014). Role of Rab11 in planar cell polarity and apical constriction during vertebrate neural tube closure. Nat. Commun. 5, 3734. 10.1038/ncomms4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipova, O., Kim, K. and Sokol, S. Y. (2015). Planar polarization of Vangl2 in the vertebrate neural plate is controlled by Wnt and Myosin II signaling. Biol. Open 4, 722-730. 10.1242/bio.201511676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, M. and Moon, R. T. (2002). The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat. Cell Biol. 4, 20-25. 10.1038/ncb716 [DOI] [PubMed] [Google Scholar]
- Peng, Y. and Axelrod, J. D. (2012). Asymmetric protein localization in planar cell polarity: mechanisms, puzzles, and challenges. Curr. Top. Dev. Biol. 101, 33-53. 10.1016/B978-0-12-394592-1.00002-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis, A. H., Xiang, B., Ossipova, O., Itoh, K. and Sokol, S. Y. (2021). Identification of the centrosomal maturation factor SSX2IP as a WTIP-binding partner by targeted proximity biotinylation. PLoS ONE 16, e0259068. 10.1371/journal.pone.0259068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux, K. J., Kim, D. I., Raida, M. and Burke, B. (2012). A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 196, 801-810. 10.1083/jcb.201112098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer, B., Onishi, K., Lo, C., Colakoglu, G. and Zou, Y. (2011). Vangl2 promotes Wnt/planar cell polarity-like signaling by antagonizing Dvl1-mediated feedback inhibition in growth cone guidance. Dev. Cell 20, 177-191. 10.1016/j.devcel.2011.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, D. L., Goisset, C. and Boucaut, J. C. (1998). Expression of Xfz3, a Xenopus frizzled family member, is restricted to the early nervous system. Mech. Dev. 70, 35-47. 10.1016/S0925-4773(97)00166-4 [DOI] [PubMed] [Google Scholar]
- Sokol, S. Y. (2015). Spatial and temporal aspects of Wnt signaling and planar cell polarity during vertebrate embryonic development. Semin. Cell Dev. Biol. 42, 78-85. 10.1016/j.semcdb.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt, D. I., Weber, U. and Mlodzik, M. (1997). The role of RhoA in tissue polarity and Frizzled signalling. Nature 387, 292-295. 10.1038/387292a0 [DOI] [PubMed] [Google Scholar]
- Strutt, H., Gamage, J. and Strutt, D. (2019). Reciprocal action of Casein Kinase Iε on core planar polarity proteins regulates clustering and asymmetric localisation. Elife 8, e45107. 10.7554/eLife.45107.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt, H., Warrington, S. J. and Strutt, D. (2011). Dynamics of core planar polarity protein turnover and stable assembly into discrete membrane subdomains. Dev. Cell 20, 511-525. 10.1016/j.devcel.2011.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tree, D. R. P., Shulman, J. M., Rousset, R., Scott, M. P., Gubb, D. and Axelrod, J. D. (2002). Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell 109, 371-381. 10.1016/S0092-8674(02)00715-8 [DOI] [PubMed] [Google Scholar]
- Turner, D. L. and Weintraub, H. (1994). Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 8, 1434-1447. 10.1101/gad.8.12.1434 [DOI] [PubMed] [Google Scholar]
- Vladar, E. K., Bayly, R. D., Sangoram, A. M., Scott, M. P. and Axelrod, J. D. (2012). Microtubules enable the planar cell polarity of airway cilia. Curr. Biol. 22, 2203-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Guo, N. and Nathans, J. (2006). The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J. Neurosci. 26, 2147-2156. 10.1523/JNEUROSCI.4698-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, C. G., Wang, B., Ballew, A., Royou, A., Karess, R., Axelrod, J. D. and Luo, L. (2001). Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105, 81-91. 10.1016/S0092-8674(01)00298-7 [DOI] [PubMed] [Google Scholar]
- Wu, J. and Mlodzik, M. (2008). The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev. Cell 15, 462-469. 10.1016/j.devcel.2008.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, W., Garrett, L., Feng, D., Elliott, G., Liu, X., Wang, N., Wong, Y. M., Choi, N. T., Yang, Y. and Gao, B. (2017). Wnt-induced Vangl2 phosphorylation is dose-dependently required for planar cell polarity in mammalian development. Cell Res. 27, 1466-1484. 10.1038/cr.2017.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, H., Copley, C. O., Goodrich, L. V. and Deans, M. R. (2012). Comparison of phenotypes between different vangl2 mutants demonstrates dominant effects of the Looptail mutation during hair cell development. PLoS ONE 7, e31988. 10.1371/journal.pone.0031988 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.