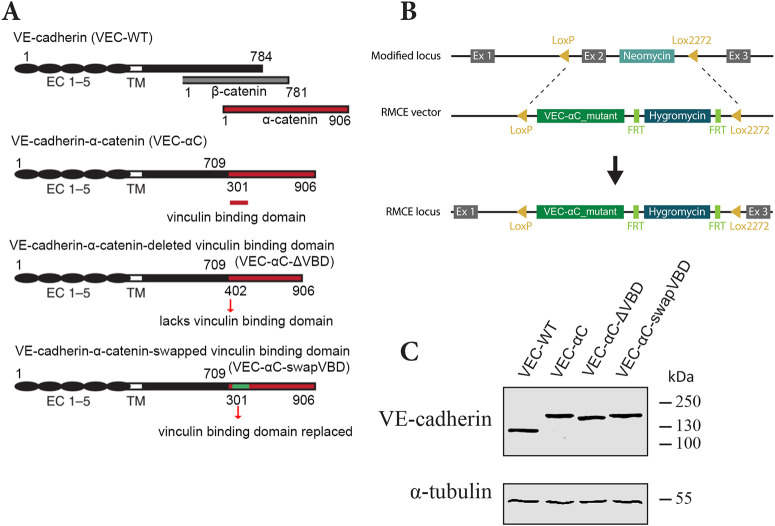
Fig. 2.
Characterization of the VEC-αC-ΔVBD and VEC-αC-swapVBD constructs. (A) Schematic illustration of VE-cadherin, β-catenin, α-catenin, and VEC-αC, VEC-αC-ΔVBD and VEC-αC-swapVBD fusion proteins. VEC-αC consists of a truncated form of VE-cadherin lacking the β-catenin-binding site (C-terminal 75 aa), which was fused to the C-terminal part (aa 301–906) of α-catenin. VEC-αC-ΔVBD and the VEC-αC-swapVBD are both unable to associate with vinculin and were constructed by modifying VEC–αC. The vinculin binding domain of α-catenin (aa 301–401) was deleted in VEC-αC-ΔVBD or was replaced by a homologous domain from mouse vinculin (green) in VEC-αC-swapVBD. Numbers refer to amino acid positions. (B) Targeting strategy for the generation of VEC–αC mutant knock-in mice by recombinase-mediated cassette exchange (RMCE). (C) MDMVECs were isolated from the four knock-in mouse lines (as indicated) and lysates were immunoblotted for VE-cadherin and α-tubulin (representative of n=2 independent experiments).