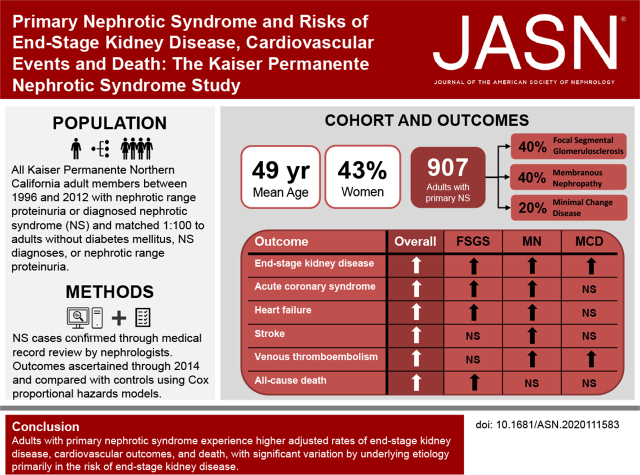
Significance Statement
Little population-based data exist about adults with primary nephrotic syndrome and risks of kidney, cardiovascular, and mortality outcomes. In a cohort of 907 adults with nephrologist-confirmed primary nephrotic syndrome attributed to minimal change disease, FSGS, and membranous nephropathy, adjusted rates of ESKD, acute coronary syndrome, heart failure, ischemic stroke, venous thromboembolism, and death were significantly higher than in 89,593 matched adults with no diabetes or known proteinuria. Adults with FSGS had the highest rate of ESKD, followed by adults with membranous nephropathy and adults with minimal change disease. Additional research is needed to understand the mechanisms underlying these excess risks, and population-level identification strategies on the basis of electronic health records should be implemented to identify and optimize personalized care of patients with primary nephrotic syndrome.
Keywords: nephrotic syndrome, end-stage kidney disease, cardiovascular events, mortality risk, heart failure, epidemiology and outcomes
Visual Abstract
Abstract
Background
Little population-based data exist about adults with primary nephrotic syndrome.
Methods
To evaluate kidney, cardiovascular, and mortality outcomes in adults with primary nephrotic syndrome, we identified adults within an integrated health care delivery system (Kaiser Permanente Northern California) with nephrotic-range proteinuria or diagnosed nephrotic syndrome between 1996 and 2012. Nephrologists reviewed medical records for clinical presentation, laboratory findings, and biopsy results to confirm primary nephrotic syndrome and assigned etiology. We identified a 1:100 time-matched cohort of adults without diabetes, diagnosed nephrotic syndrome, or proteinuria as controls to compare rates of ESKD, cardiovascular outcomes, and death through 2014, using multivariable Cox regression.
Results
We confirmed 907 patients with primary nephrotic syndrome (655 definite and 252 presumed patients with FSGS [40%], membranous nephropathy [40%], and minimal change disease [20%]). Mean age was 49 years; 43% were women. Adults with primary nephrotic syndrome had higher adjusted rates of ESKD (adjusted hazard ratio [aHR], 19.63; 95% confidence interval [95% CI], 12.76 to 30.20), acute coronary syndrome (aHR, 2.58; 95% CI, 1.89 to 3.52), heart failure (aHR, 3.01; 95% CI, 2.16 to 4.19), ischemic stroke (aHR, 1.80; 95% CI, 1.06 to 3.05), venous thromboembolism (aHR, 2.56; 95% CI, 1.35 to 4.85), and death (aHR, 1.34; 95% CI, 1.09 to 1.64) versus controls. Excess ESKD risk was significantly higher for FSGS and membranous nephropathy than for presumed minimal change disease. The three etiologies of primary nephrotic syndrome did not differ significantly in terms of cardiovascular outcomes and death.
Conclusions
Adults with primary nephrotic syndrome experience higher adjusted rates of ESKD, cardiovascular outcomes, and death, with significant variation by underlying etiology in the risk for developing ESKD.
Nephrotic syndrome (NS) describes the clinical manifestations of several rare kidney diseases, which are generally characterized clinically by the presence of peripheral edema, heavy proteinuria, hypoalbuminemia, and hypercholesterolemia.1 Most adult patients with NS have primary glomerular diseases, including FSGS, membranous nephropathy (MN), and minimal change disease (MCD).1,2 Although the population incidence of NS is estimated to be approximately 3 per 100,000 person-years, NS has been associated with a range of adverse outcomes including systemic infection, cardiovascular and thromboembolic events, and AKI. However, few studies have been large enough or conducted in broadly diverse, enumerated populations to accurately estimate risks.1–3 Given the potential for serious clinical complications in patients with NS, population-level identification methods and effective disease management strategies could help physicians and health systems to optimize clinical and patient-centered outcomes.
Prospective cohorts, registries, and other retrospective studies have provided valuable insights into the etiology, incidence, and outcomes of patients with confirmed NS.4–13 However, these studies often required biopsy-confirmed NS for study inclusion, relied only on diagnostic coding, included both primary and secondary NS, and only data from tertiary care medical centers, had limited sociodemographic diversity, or lacked long-term follow-up. As a result, existing estimates of incidence and outcome burden may not accurately reflect the broader population of patients with primary NS, overall and by presumed underlying cause.
To address these knowledge gaps, we leveraged data from a large, integrated health care delivery system to identify and follow adults with primary NS over a 16-year period, and compared long-term kidney and cardiovascular outcomes, overall and by classified etiology, with a population of adults without documented diabetes or NS.
Methods
Source Population and Study Sample
The source population included members of Kaiser Permanente Northern California (KPNC), an integrated health care delivery system providing comprehensive care to >4.5 million members throughout Northern California. KPNC membership is highly representative of the regional and statewide population in terms of sociodemographic characteristics.14
We first aimed to identify a cohort with confirmed primary NS. We initially included all adult (age ≥18 years) KPNC members who had nephrotic-range proteinuria or a diagnosis of NS between January 1, 1996 and December 31, 2012. Eligible patients with potential primary NS were identified using clinically obtained laboratory test results and/or diagnosis codes found in electronic health records (EHR); details of specific methods are outlined below. After identifying the initial cohort with potential primary NS, targeted subgroups were selected for adjudication by board-certified nephrologists using medical records and standardized criteria to confirm the presence of NS, type of NS (primary versus secondary), and definite (i.e., biopsy proven) or presumed cause of primary NS (FSGS, MN, or MCD).
After identifying adults with confirmed primary NS, we individually matched (using a 1:100 matching ratio) adults without diabetes mellitus (DM), NS, or evidence of nephrotic range proteinuria using a prespecified matching method without replacement. Patients without any documented nephrotic range proteinuria or DM were included in the eligible control population. Diabetes was defined as having ≥1 primary hospital discharge diagnosis for DM, ≥2 outpatient diagnoses for DM, ≥2 abnormal laboratory results (fasting glucose, random glucose, or hemoglobin A1C) on separate days, or ≥1 outpatient prescriptions for insulin or hypoglycemic agents; and no diagnosed gestational diabetes. We required that matched control patients meet the following additional inclusion criteria: alive and an active health plan member on the index date of the matched NS patient; date of birth within 1 month of the matched patient with NS; ≥12 months of continuous KPNC membership before the matched NS patient’s index date; ≥1 outpatient visit within the 12 months before the NS index date; and ≥1 outpatient serum creatinine laboratory measurement in the 12 months before index date of the matched patient with NS. We excluded all patients with prior ESKD, defined as receipt of maintenance dialysis or kidney transplant identified from a comprehensive ESKD treatment registry.15
The study was approved by the KPNC institutional review board, and waiver of informed consent was obtained due to the nature of the study.
Identification of Potential NS Using Proteinuria Results and Diagnosis Codes
We identified adults with nephrotic-range proteinuria found in health system laboratory databases and defined as either urine albumin-creatinine ratio >3500 ug/mg, protein-creatinine ratio >3.5 mg/mg, or 24-hr urine protein excretion >3500 mg. We also identified all adults who had ≥1 primary hospital discharge, emergency department, or outpatient encounter diagnosis code of NS during the inception period. We ascertained NS diagnoses from comprehensive health plan databases on the basis of relevant International Classification of Diseases, Ninth Edition codes (581, 581.0, 581.1, 581.2, 581.3, 581.8, 581.81, 581.89, and 581.9).
On the basis of the combination of proteinuria findings and presence of NS diagnosis codes, we prioritized individual review of medical records by board-certified nephrologists as follows: (1) nephrotic range proteinuria with diagnoses of NS and a primary NS etiology, (2) nephrotic range proteinuria with diagnoses of NS and other NS etiology, (3) nephrotic range proteinuria with a diagnosis of primary NS etiology alone, (4) nephrotic range proteinuria and an NS diagnosis without stated etiology, (5) no documented proteinuria, but with diagnosis of NS and primary NS etiology, (6) significant proteinuria (urine albumin-creatinine ratio 3000–3499 mg/g) with diagnosis of NS and other NS etiology, (7) significant proteinuria with diagnosis of primary NS etiology alone, (8) no documented proteinuria but with a generalized NS diagnosis, and (9) nephrotic range proteinuria with no associated renal-related diagnosis. A detailed flow diagram of eligible patients and prioritized adjudicated patient subgroups are shown in Supplemental Figure 1, and a description of the review criteria can be found in the Supplemental Document.
Validation of NS and Analytic Cohort Assembly
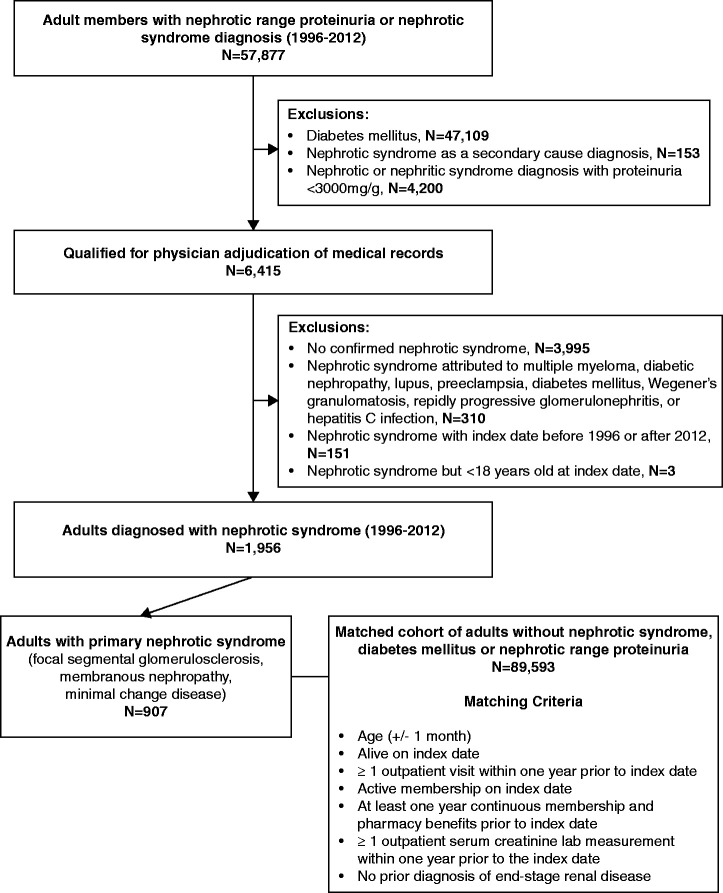
A total of 6415 adults met the initial eligibility criteria for potential NS described above, and their records underwent physician adjudication (Figure 1). Using combined data from the EHR and manual review of medical records, confirmed NS required evidence of symptoms and/or signs consistent with NS and a laboratory measurement confirming nephrotic-range proteinuria at the time of diagnosis. We ascertained the cause of NS by review of biopsy results, if available. For patients without available biopsy results, we incorporated relevant information from nephrology or other treating physician notes to assign the presumed etiology. Causes of NS assigned using an available biopsy result found in KPNC databases were considered “definite;” all others were considered “presumed.”
Figure 1.
Cohort assembly of adults with primary NS and matched adults without NS identified between 1996 and 2012.
We excluded patients if the physician reviewers could not confirm a diagnosis of NS or among those with confirmed NS, having an index date (i.e., date of initial NS diagnosis) that could not be established, having an index date was before 1996 or after 2012, or being younger than 18 years on the index date.
Covariates
We obtained demographic characteristics (age, sex, self-reported race, and Hispanic ethnicity, if available) from health plan databases. We defined relevant comorbidities by diagnosis or procedure codes supplemented with laboratory test results, outpatient vital signs, and/or prescribed medications using EHR-based data that were cleaned using standardized procedures and linked at the individual-patient level in the Kaiser Permanente Virtual Data Warehouse as previously described and validated.16,17
Follow-up and Outcomes
We censored follow-up time at death, loss of health plan membership, or December 31, 2014, whichever was earliest. We ascertained ESKD through a validated health plan registry.15 Cardiovascular outcomes included hospitalization for acute coronary syndrome (ACS) (defined as acute myocardial infarction [MI] or unstable angina), heart failure, ischemic stroke, and venous thromboembolism (VTE), using previously validated International Classification of Diseases, Ninth Edition diagnosis codes for hospitalizations occurring in KPNC and non-network facilities that were comprehensively captured in our EHR. Death was ascertained on the basis of comprehensive information from health plan administrative and clinical databases, member proxy reporting, Social Security Administration vital status files, and state death certificate information.18
Statistical Analyses
We conducted all analyses using SAS version 9.3 (Cary, NC). We first characterized the primary NS cohort on the basis of assigned etiology (Supplemental Table 1) and calculated overall incidence rates of primary NS per 100,000 individuals among the total nondiabetic adult population at KPNC from 1996 to 2012. Given the large overall sample size, we next compared characteristics among patients with primary NS and matched controls using standard differences in each variable by calculating a difference in means of the two groups divided by the pooled standard deviation. This value reflects a standardized magnitude of the difference between groups comparable across characteristics with varying distributions; for example, a value of 0.10 would indicate a 10% difference between groups.
For each outcome of interest, we calculated crude rates per 100 person-years and associated 95% confidence intervals (95% CI) for adults with primary NS and matched controls. We then conducted multivariable Cox proportional hazard regression models adjusted for baseline covariates including demographic characteristics (age, sex, race, Hispanic ethnicity, smoking status, socioeconomic status), comorbidities (acute myocardial infarction, unstable angina, ischemic stroke, transient ischemic attack, peripheral artery disease, dyslipidemia, heart failure, hypertension, hyperthyroidism, hypothyroidism, liver disease, lung disease, cancer, dementia), and targeted laboratory results (eGFR, hemoglobin) to evaluate the association between primary NS and the first occurrence of outcomes of interest, with reported adjusted hazard ratios and 95% CIs. For each model, we evaluated the proportional hazards assumption by examining log-log plots. Because matching was conducted based solely on age and prior resource utilization dates to obtain an at-risk control group, we did not preserve the matching in the Cox models. We also examined multivariable associations stratified by type of primary NS (FSGS, MN, or MCD) for each outcome of interest. In a sensitivity analysis to assess the importance of subsequent changes in comorbidity burden with ESKD incidence, we conducted extended Cox regression models for NS with time-updated age and comorbidities. Finally, to compare rates between etiologies of primary NS, we conducted a multivariable Cox proportional hazards model among patients with NS only, and report adjusted hazard ratios and 95% CIs for each etiology group comparison.
Results
Cohort Assembly, Characteristics, and Follow-up
Among 6415 prioritized eligible adults with potential NS that underwent physician adjudication of medical records, we identified 1956 adults with confirmed NS, of whom 1070 (54.7%) had available renal biopsy results (Table 1). Among the 1956 adults with confirmed NS, we identified 907 (46%) with primary NS (Supplemental Figure 2); of note, 655 (72%) of those with primary NS had available biopsy results. The estimated incidence of primary NS over the study period among nondiabetic adults was 2.60 per 100,000 individuals. Among adults with primary NS, 359 (40%) had FSGS, 366 (40%) had MN, and 182 (20%) had MCD.
Table 1.
Proportion of confirmed patients with NS by chart review priority group
| Identification Approach | Chart Review Priority Group | Total Charts Reviewed | Confirmed NS, n (%) |
|---|---|---|---|
| NS diagnosis code with MCD, FSGS, or MN diagnosis code and documented nephrotic proteinuria | 1 | 88 | 82 (93.2) |
| NS diagnosis code with diagnosis code for other cause and documented nephrotic proteinuria | 2 | 537 | 288 (53.6) |
| MCD, FSGS, or MN diagnosis code alone and documented nephrotic proteinuria | 3 | 148 | 124 (83.8) |
| NS diagnosis code with no listed cause and documented nephrotic proteinuria | 4 | 153 | 91 (59.4) |
| NS diagnosis code with MCD, FSGS, or MN diagnosis code and no documented nephrotic proteinuria | 5 | 222 | 162 (73.0) |
| NS diagnosis code with diagnosis code for other cause and documented urine albumin-creatinine ratio between 3000–3499 mg/g | 6 | 1115 | 517 (46.4) |
| MCD, FSGS, or MN diagnosis code alone and documented urine albumin-creatinine ratio between 3000–3499 mg/g | 7 | 349 | 247 (70.8) |
| NS diagnosis code with no listed cause | 8 | 1230 | 202 (16.4) |
| No renal diagnosis but with documented proteinuria from urine albumin-creatinine ratio, protein-creatinine ratio, or 24-hour urine protein excretion measurements | 9 | 2573 | 243 (9.4) |
We next matched the 907 adults with definite or presumed primary NS to 89,593 healthy adults with no documented evidence of DM, diagnosed NS, or evidence of nephrotic range proteinuria (Figure 1). Compared with matched controls, patients with primary NS were more likely to be men, be persons of color, have a higher comorbidity burden (i.e., prior MI, heart failure, ischemic stroke, hypertension, dyslipidemia), have higher body mass index and blood pressure, have a higher total cholesterol level, and lower baseline eGFR, serum albumin, and hemoglobin (Table 2). The median follow-up for subsequent outcomes was 4.5 (interquartile range, 2.2–8.4) years.
Table 2.
Baseline characteristics of adults with primary NS and matched adults without NS identified between 1996–2012
| Characteristic | Adults with Primary NS | Adults without NS | Standardized Difference |
|---|---|---|---|
| (n=907) | (n=89,593) | ||
| Demographic characteristics | |||
| Mean (SD) age, yr | 49.0 (16.8) | 49.2 (16.7) | 0.01 |
| Women, n (%) | 390 (43.0) | 50,857 (56.8) | 0.28 |
| Self-reported race, n (%) | 0.12 | ||
| White/European | 375 (41.3) | 49,119 (54.8) | |
| Black | 131 (14.4) | 6277 (7.0) | |
| Asian/Pacific Islander | 166 (18.3) | 12,025 (13.4) | |
| Other | 75 (8.3) | 4970 (5.5) | |
| Unknown | 160 (17.6) | 17,202 (19.2) | |
| Hispanic ethnicity, n (%) | 176 (19.4) | 12,571 (14.0) | 0.15 |
| Low household educational attainment, n (%) | 226 (24.9) | 17,537 (19.6) | 0.13 |
| Median household income <$35,000, n (%) | 104 (11.5) | 8956 (10.0) | 0.05 |
| Current or former smoker, n (%) | 198 (21.8) | 19,063 (21.3) | 0.01 |
| Medical history, n (%) | 4 (1.1) | ||
| Myocardial infarction | 10 (1.1) | 518 (0.6) | 0.39 |
| Unstable angina | 6 (0.7) | 347 (0.4) | 0.33 |
| Heart failure | 25 (2.8) | 915 (1.0) | 0.61 |
| Ischemic stroke | 5 (0.6) | 236 (0.3) | 0.45 |
| Transient ischemic attack | 8 (0.9) | 384 (0.4) | 0.44 |
| Venous thromboembolism | 3 (0.3) | 148 (0.2) | 0.22 |
| Peripheral artery disease | 8 (0.9) | 434 (0.5) | 0.37 |
| Hypertension | 368 (40.6) | 20,571 (23.0) | 0.50 |
| Dyslipidemia | 370 (40.8) | 20,589 (23.0) | 0.51 |
| Chronic liver disease | 13 (1.4) | 1470 (1.6) | 0.08 |
| Chronic lung disease | 139 (15.3) | 12,214 (13.6) | 0.08 |
| Asthma | 119 (13.1) | 10,257 (11.4) | 0.09 |
| Hyperthyroidism | 25 (2.8) | 1929 (2.2) | 0.15 |
| Hypothyroidism | 116 (12.8) | 7619 (8.5) | 0.28 |
| Diagnosed dementia | 3 (0.3) | 617 (0.7) | 0.45 |
| Cancer | 40 (4.4) | 5278 (5.9) | 0.18 |
| Cardiac procedure history, n (%) | |||
| Coronary artery bypass graft surgery | 5 (0.6) | 355 (0.4) | 0.20 |
| Percutaneous coronary intervention | 14 (1.5) | 645 (0.7) | 0.47 |
| Medication use, n (%) | |||
| ACE inhibitor | 229 (25.2) | 9329 (10.4) | 0.65 |
| Angiotensin II receptor blocker | 67 (7.4) | 1760 (2.0) | 0.84 |
| Beta-blocker | 180 (19.8) | 10,522 (11.7) | 0.38 |
| Calcium channel blocker | 130 (14.3) | 4520 (5.0) | 0.70 |
| Diuretic | 371 (40.9) | 13,585 (15.2) | 0.82 |
| Alpha antagonist | 72 (7.9) | 2687 (3.0) | 0.62 |
| Aldosterone receptor antagonist | 13 (1.4) | 419 (0.5) | 0.68 |
| Other antihypertensive agent | 522 (57.6) | 25,590 (28.6) | 0.74 |
| Antiarrhythmic agent | 1 (0.1) | 391 (0.4) | 0.84 |
| Digoxin | 9 (1.0) | 984 (1.1) | 0.06 |
| Statin | 224 (24.7) | 11,016 (12.3) | 0.52 |
| Other lipid-lowering agent | 35 (3.9) | 1166 (1.3) | 0.67 |
| Warfarin | 28 (3.1) | 1616 (1.8) | 0.33 |
| Platelet aggregation inhibitor | 10 (1.1) | 662 (0.7) | 0.24 |
| Nonsteroidal anti-inflammatory drug | 117 (12.9) | 13,777 (15.4) | 0.12 |
| Vital signs | |||
| Body mass index, kg/m2, n (%) | 0.12 | ||
| <18.5 | 7 (0.8) | 655 (0.7) | |
| 18.5–25.0 | 110 (12.1) | 16,502 (18.4) | |
| 25.0–29.9 | 157 (17.3) | 17,116 (19.1) | |
| 30.0–39.9 | 195 (21.5) | 14,975 (16.7) | |
| ≥40.0 | 27 (3.0) | 1553 (1.7) | |
| Unknown | 411 (45.3) | 38,792 (43.3) | |
| Systolic blood pressure, mmHg, n (%) | 0.33 | ||
| <120 | 135 (14.9) | 29,377 (32.8) | |
| 121–130 | 110 (12.1) | 13,774 (15.4) | |
| 131–139 | 174 (19.2) | 14,804 (16.5) | |
| 140–159 | 153 (16.9) | 7235 (8.1) | |
| 160–179 | 48 (5.3) | 1428 (1.6) | |
| ≥180 | 20 (2.2) | 298 (0.3) | |
| Unknown | 267 (29.4) | 22,677 (25.3) | |
| Diastolic blood pressure, mmHg, n (%) | 0.24 | ||
| ≤80 | 372 (41.0) | 50,478 (56.3) | |
| 81–84 | 71 (7.8) | 6944 (7.8) | |
| 85–89 | 83 (9.2) | 5283 (5.9) | |
| 90–99 | 69 (7.6) | 3371 (3.8) | |
| 100–109 | 29 (3.2) | 697 (0.8) | |
| ≥ 110 | 15 (1.7) | 108 (0.1) | |
| Unknown | 268 (29.5) | 22,712 (25.4) | |
| Laboratory values | |||
| Estimated glomerular filtration rate, ml/min per 1.73m2 | 2.01 | ||
| ≥90 | 268 (29.5) | 46,809 (52.2) | |
| 60–89 | 176 (19.4) | 35,990 (40.2) | |
| 45–59 | 106 (11.7) | 4864 (5.4) | |
| 30–44 | 112 (12.3) | 1404 (1.6) | |
| 15–29 | 106 (11.7) | 256 (0.3) | |
| <15 | 37 (4.1) | 27 (0.0) | |
| Unknown | 102 (11.2) | 243 (0.3) | |
| Serum albumin, mg/dl | |||
| Mean (SD) | 2.8 (0.9) | 4.3 (0.4) | 2.95 |
| Missing, n (%) | 382 (42.1) | 81,310 (90.8) | |
| Total cholesterol, mg/dl | 0.47 | ||
| <200 | 149 (16.4) | 32,194 (35.9) | |
| 200–240 | 99 (10.9) | 20,558 (22.9) | |
| >240 | 361 (39.8) | 9,304 (10.4) | |
| Unknown | 298 (32.9) | 27,537 (30.7) | |
| Hemoglobin, g/dl | 0.24 | ||
| >13.0 | 460 (50.7) | 56,989 (63.6) | |
| 12.0–12.9 | 107 (11.8) | 11,756 (13.1) | |
| 11.0–11.9 | 78 (8.6) | 4061 (4.5) | |
| 10.0–10.9 | 44 (4.9) | 1289 (1.4) | |
| 9.0–9.9 | 22 (2.4) | 466 (0.5) | |
| <9.0 | 11 (1.2) | 261 (0.3) | |
| Unknown | 185 (20.4) | 14,771 (16.5) |
Primary NS and ESKD
During follow-up, 369 patients developed ESKD in the matched cohort, with a higher crude rate (4.65; 95% CI, 4.08 to 5.31 per 100 person-years) in those with primary NS compared with matched controls (0.03; 95% CI, 0.03 to 0.04 per 100 person-years) (Table 3 and Supplemental Figure 3).
Table 3.
Crude rates per 100 person-years (95% CI) of ESKD, cardiovascular outcomes, and death among adults with primary NS and matched adults without NS
| Outcomes | Adults with Primary NS | Matched Adults without NS | ||
|---|---|---|---|---|
| n=907 | n=89,593 | |||
| Number of Events | Rate per 100 Person-Years (95% CI) | Number of Events | Rate per 100 Person-Years (95% CI) | |
| ESKD | 218 | 4.65 (4.08 to 5.31) | 151 | 0.03 (0.03 to 0.04) |
| ACS | 56 | 1.02 (0.78 to 1.32) | 1420 | 0.28 (0.27 to 0.30) |
| Heart failure | 55 | 0.99 (0.76 to 1.29) | 885 | 0.18 (0.16 to 0.19) |
| Ischemic stroke | 19 | 0.33 (0.21 to 0.52) | 740 | 0.15 (0.14 to 0.16) |
| Venous thromboembolism | 14 | 0.25 (0.15 to 0.42) | 302 | 0.06 (0.05 to 0.07) |
| Death from any cause | 136 | 2.37 (2.00 to 2.80) | 6823 | 1.35 (1.32 to 1.38) |
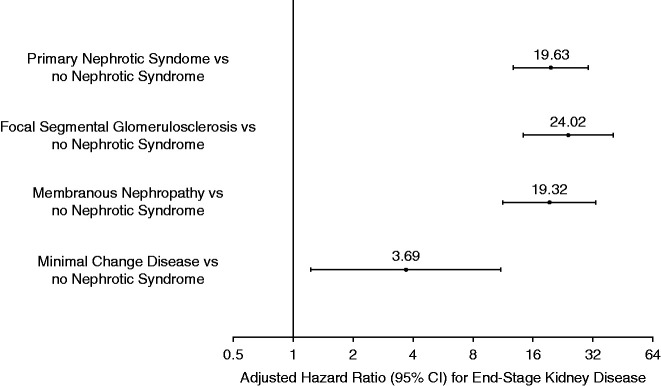
In multivariable models, primary NS was associated with a nearly 20-fold higher adjusted rate of ESKD (adjusted hazard ratio [aHR], 19.63; 95% CI, 12.76 to 30.20) (Figure 2). The excess risk of ESKD varied by etiology of primary NS; FSGS was associated with the highest adjusted rate of ESKD (aHR, 24.02; 95% CI, 14.25 to 40.47), followed by MN (aHR, 19.32; 95% CI, 11.26 to 33.15) and MCD (aHR, 3.39; 95% CI, 1.23 to 11.01) compared with matched controls (Figure 2). Significant differences in ESKD risk between etiologies of primary NS were also observed (Table 4). In a sensitivity analysis, incorporating time-updated age and comorbidities only slightly attenuated the association between primary NS and ESKD (Supplemental Table 2).
Figure 2.
Multivariable association between primary NS and risk of developing ESKD. Models adjusted for age, sex, race, ethnicity, smoking status, socioeconomic status, baseline comorbidities, and laboratory values.
Table 4.
Multivariable pairwise associations between etiology of primary NS groups and outcomes. All models adjusted for age, sex, race, ethnicity, smoking status, socioeconomic status, baseline comorbidities and laboratory values
| Adjusted Hazard Ratios (95% Confidence Intervals) | ||||||
|---|---|---|---|---|---|---|
| Comparison | ESKD | Venous Thromboembolism | ACS | Ischemic Stroke | Heart Failure | Death |
| FSGS versus MCD | 9.75 (3.71 to 25.59) | 0.25 (0.05 to 1.12) | 2.99 (0.94 to 9.51) | 0.97 (0.17 to 5.40) | 3.42 (0.60 to 19.42) | 1.66 (0.83 to 3.31) |
| MN versus MCD | 5.72 (2.15 to 15.21) | 0.75 (0.15 to 3.74) | 2.22 (0.66 to 7.40) | 2.39 (0.65 to 8.74) | 2.59 (0.43 to 15.68) | 1.25 (0.62 to 2.51) |
| FSGS versus MN | 1.71 (1.18 to 2.46) | 0.33 (0.08 to 1.30) | 1.35 (0.61 to 2.98) | 0.41 (0.09 to 1.79) | 1.32 (0.60 to 2.92) | 1.33 (0.87 to 2.02) |
Primary NS, Cardiovascular Outcomes, and Death
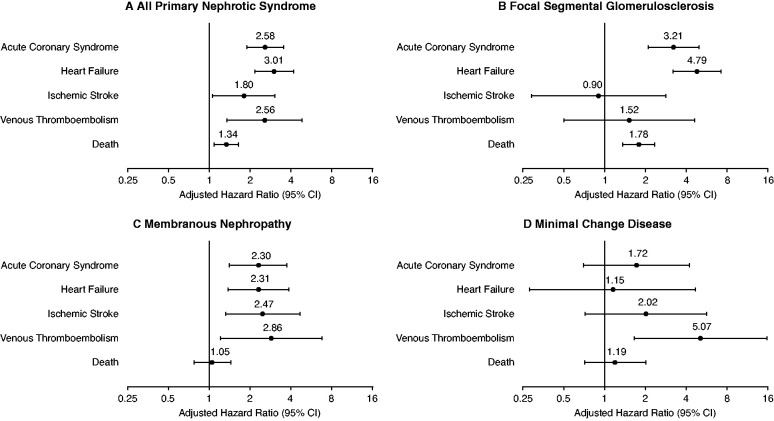
A total of 1476 patients experienced a subsequent ACS event in the matched cohort during follow-up, with a higher crude rate in those with primary NS compared with matched controls (Table 3). In multivariable analysis, adults with primary NS had a more than 2.5-fold higher adjusted rate of incident ACS compared with matched controls (aHR, 2.58; 95% CI, 1.89 to 3.52) (Figure 3).
Figure 3.
Multivariable associations between primary NS and subsequent cardiovascular outcomes and death from any cause. All models adjusted for age, sex, race, ethnicity, smoking status, socioeconomic status, baseline comorbidities, and laboratory values.
During follow-up, 940 adults were hospitalized for heart failure, with higher crude rates among patients with primary NS (Table 3). After adjustment for potential confounders, primary NS was associated with a three-fold higher adjusted rate of heart failure compared with matched controls (aHR, 3.01; 95% CI, 2.16 to 4.19) (Figure 3).
Overall, 759 patients experienced an ischemic stroke, and those with primary NS experienced a higher crude rate compared with matched controls (Table 3). In multivariable analysis, primary NS was associated with a nearly two-fold higher adjusted rate of ischemic stroke (aHR, 1.80; 95% CI, 1.06 to 3.05) (Figure 3).
A total of 316 patients suffered a VTE event in the overall matched cohort during follow-up, with a significantly higher rate in patients with primary NS compared with matched controls (Table 3). After adjustment for potential confounders, patients with primary NS had a more than 2.5-fold higher adjusted rate of VTE than matched controls (aHR, 2.56; 95% CI, 1.35 to 4.85) (Figure 3).
During follow-up, 6959 patients died, with a higher crude rate in patients with primary NS compared with matched controls (Table 3). In multivariable analysis, compared with matched controls, those with primary NS had a 34% higher adjusted rate of death from any cause (aHR, 1.34; 95% CI, 1.09 to 1.64) (Figure 3).
Finally, Figure 3 shows relative risks of cardiovascular outcomes in aggregate and by etiology of primary NS. Although the point estimates for risks of death, ACS, and heart failure were highest for those with FSGS, the point estimate for risk of ischemic stroke was highest for MN and highest for MCD for VTE, but there were no statistically significant differences between etiologies of primary NS (Figure 3 and Table 4).
Discussion
By leveraging a combination of comprehensive EHR data and physician adjudication of medical records, we identified 907 adults with primary NS receiving longitudinal care within an integrated health care delivery system over a 16-year period. Over a median follow-up of 4.5 years, adults with primary NS had a nearly 20-fold higher adjusted rate of ESKD, and higher adjusted rates of multiple types of cardiovascular outcomes and all-cause death compared with a large cohort of matched adults without diabetes, diagnosed NS, or known evidence of nephrotic range proteinuria. Importantly, we also found a significant variation in the adjusted rate of ESKD by etiology of primary NS. Our methodological approach to systematically identifying adults with primary NS and the demonstration of the high associated excess risks of adverse kidney and cardiovascular outcomes within primary NS in this diverse, community-based population support the need to design more effective population-based surveillance and care delivery strategies for these patients who are high risk.
Our study findings support and materially extend results from previous studies that investigated outcomes in adults with NS. For example, in a prospective study in 580 Chinese adults undergoing kidney biopsy and in an analysis of data among patients receiving renal replacement therapy from the United States Renal Data System, FSGS was reported as the leading cause of ESKD caused by primary glomerular disease.5,19 In our study among adults with primary NS, we found comparably high adjusted ESKD rates in those with either FSGS or MN, suggesting a much higher risk of developing ESKD in patients with MN than previously observed.20
VTE is considered one of the more serious complications associated with NS,3,21 but its incidence has not been well defined in comparison to control patients. Depending on the location of the VTE and the detection strategy used, the cumulative risk of incident VTE associated with any NS has been reported to be between 3% and 48%.22–24 Our study defined incident VTE as hospitalization for deep vein thrombosis, pulmonary embolism, or renal vein thrombosis, and observed an annual incidence of 0.25% in those with primary NS. We found that those with primary NS had a more than 2.5-fold higher adjusted risk of VTE compared with matched controls without NS, but no significant differences between etiologies of primary NS. The latter finding is consistent with certain previous studies,25,26 but not others.21,23,27
The risk of other types of cardiovascular events associated with NS has been less well studied.28,29 An earlier analysis within KPNC by Ordoñez et al. examined a cohort of 142 adults with primary or secondary NS and 142 age- and sex-matched controls identified between 1976 and 1981, and followed for MI and death attributed to coronary disease through to 1987.29 That study observed significantly higher adjusted risks of MI (adjusted relative risk, 5.5; 95% CI, 1.6 to 18.3) but not death attributed to coronary disease (relative risk, 2.8; 95% CI, 0.7 to 11.3). Mahmoodi et al. investigated the risk of MI, unstable angina, and ischemic stroke in 298 Dutch adults with primary or secondary NS, and reported an annual incidence of arterial thromboembolic events of 1.48%, with MI and unstable angina much more common than peripheral artery disease or ischemic stroke, but there was no comparison group.30 We observed an annual incidence of 1.02% for ACS with a >2.5-fold higher adjusted rate in those with primary NS compared with matched controls. We also found an annual incidence of 0.33% for ischemic stroke, and primary NS was associated with a 1.8-fold higher adjusted rate compared with matched controls. The latter extends findings from Huang et al., who analyzed data from the National Health Insurance Research Database in Taiwan and found that the risk of ischemic stroke was higher in adults with diagnosed NS (adjusted hazard ratio, 1.38; 95% CI, 1.21 to 1.57) compared with controls matched on age, sex, and Charlson Comorbidity Index.31 Although certain symptoms and signs of NS overlap with those seen in acute heart failure (e.g., dyspnea, peripheral edema, pleural effusion), very limited data exist about the subsequent risk of hospitalization for heart failure after developing primary NS. Our study provides new insights into the excess risk of heart failure hospitalization with primary NS.
The exact mechanisms contributing to increased venous and arterial thrombosis with NS have not been well delineated, with several potential pathways that may contribute to a prothrombotic environment. Because NS leads to substantial proteinuria, there is concurrent loss of fibrinolytic factors (e.g., protein C and S, antithrombin III, plasmin, and plasminogen).32 In addition, there may be enhanced activation of platelets and platelet aggregation,33 and elevated levels of prothrombotic factors (e.g., fibrinogen) and inhibition of the activation of plasminogen.32,34 Even less is known about possible distinct pathways that may explain differences in these outcomes by underlying etiology of primary NS.
Our study also extends results related to all-cause mortality from the earlier matched cohort analysis within KPNC, which observed a higher adjusted risk of death with primary and nondiabetic secondary NS (adjusted relative risk, 7.2; 95% CI, 3.6 to 14.2) compared with controls between 1976 and 1987.29 Of note, our cohort was considerably larger and only included patients with primary NS attributed to definite or presumed FSGS, MN, or MCD in a more recent era (1996–2012). Our observed lower rate of death (2.37 per 100 person-years) compared with the earlier study can be explained, at least in part, by availability of more effective therapies for NS and cardiopreventive agents and approaches.30 Nonetheless, there remained an excess risk of death with primary NS that persisted even after accounting for differences in demographic characteristics, cardiovascular risk factors, and other comorbidities.
A strength of our study is the analysis of a large and ethnically diverse adult population receiving care in a fully integrated health care delivery system, where care is coordinated across all inpatient, emergency department, and outpatient settings, and captured through a single EHR system. This allowed for the assessment of laboratory data across all practice settings to systematically screen for adults with documented evidence of nephrotic range proteinuria, and the determination of baseline demographic and clinical characteristics. Our study was likely more representative of the adult population with primary NS because a kidney biopsy was not required for inclusion; instead, board-certified nephrologists conducted case-by-case review of relevant medical records to confirm the presence of NS and definite or presumed etiology, which is considered the gold standard for retrospective patient identification. We also examined the risks of ESKD, venous and arterial cardiovascular events, heart failure, and long-term survival to provide a more complete assessment of clinical outcomes.
Our study also had several limitations. Because we relied on data collected as part of routine clinical care across an extended time period, comprehensive data on selected variables of interest were not available. The lack of comprehensive biopsy reports in KPNC EHRs and pathology databases may result in misclassification of assigned etiologies. However, because most patients (72%) did have a pathologically confirmed NS etiology, and the remaining 28% of patients were assigned a presumed etiology by nephrologist reviewers through clinical presentation, laboratory results, nephrology notes, and notes and/or biopsy reports imported into the KPNC EHR from other health systems, we believe this misclassification is likely small and reflective of assessments made in typical clinical practice. Although the manual review of medical records and available biopsy results by nephrologists is still considered the gold standard for retrospective identification of patients with NS, it could be challenging to perform this time- and resource-intensive process on a large scale in other less integrated care settings. When identifying the control population to match with patients with primary NS, we classified the subset of patients who did not have any urine protein test results as not having nephrotic range proteinuria. Although this may result in residual confounding in analysis due to unmeasured proteinuria, we believe the likelihood these patients had undetected nephrotic range proteinuria was low and any misclassification would tend to bias results toward the null. We were also unable to directly address mechanisms underlying the excess risks of adverse kidney and cardiovascular outcomes and all-cause mortality, overall and by underlying etiology of primary NS. Due to the lack of complete, detailed longitudinal data on specific therapies for primary NS and treatment response, and the use and response to cardiovascular preventive strategies, we were unable to evaluate their contribution to clinical outcomes but this should be addressed in future studies. Finally, despite our large, diverse analyzed population, our findings may not be fully generalizable to other geographic areas, health systems, or patients who are uninsured.
In conclusion, our findings have important clinical implications at the population and individual patient level. First, we developed a population-based approach on the basis of EHR data combined with targeted physician adjudication to identify and characterize long-term outcomes of adults with primary NS. This approach could be applied by other health systems to identify adults with primary NS, and future efforts leveraging validated natural language processing algorithms and other machine-learning methods on unstructured EHR data could facilitate even more efficient population-level identification of primary NS, by avoiding the need for manual adjudication.35,36 Second, the substantial excess risk of ESKD with primary NS and particularly in those with FSGS or MN highlight the need for careful surveillance in these patients, optimizing control of risk factors for kidney disease progression, and identification of novel etiology-specific therapies. Finally, the higher risks of VTE, atherosclerotic cardiovascular events, and heart failure hospitalizations with primary NS that did not significantly vary by etiology of primary NS reinforce the importance of systematic evaluation for and management of cardiovascular risk factors and aggressive secondary prevention in those with established cardiovascular disease.
Disclosures
A. Go reports being employed by The Permanente Medical Group (TPMG) and reports receiving research funding from Amarin, Bristol Meyers-Squibb, CSL Behring, and Novartis. T. Tan, D. Fan, J. Yang, and R. Parikh are employed by KPNC. G. Chertow reports having consultancy agreements with Akebia, Amgen, Ardelyx, AstraZeneca, Baxter, Cricket, DiaMedica, Gilead, Miromatrix, Reata, Sanifit, Unicycive, and Vertex; reports having an ownership interest in Ardelyx, CloudCath, Durect, DxNow, Eliaz Therapeutics, Outset, Physiowave, and PuraCath; reports receiving research funding from National Institute of Diabetes and Digestive and Kidney Diseases and National Institute of Allergy and Infectious Diseases; reports being a scientific advisor or membership as member of the Board of Directors of Satellite Healthcare, Co-Editor of Brenner & Rector's The Kidney (Elsevier); and reports having other interests/relationships with Data and Safety Monitoring Board service: Angion, Bayer, National Institute of Diabetes and Digestive and Kidney Diseases, and ReCor. J. Ordonez reports having employment, consultancy agreements, and receiving honoraria from Satellite Healthcare. J. Wojcicki reports other interests/relationships via a family association with the Wojcicki Foundation. K. Chen is employed by TPMG. S. Zheng is employed by TPMG; and reports being scientific advisor or member as Medical Director for Wellbound Emeryville. L. Yankulin is employed by TPMG and reports other interests/relationships as Medical Director at DaVita Golden Gate Dialysis Unit. All remaining authors have nothing to disclose.
Funding
This work was supported by the Brin Wojcicki Foundation.
Supplementary Material
Acknowledgments
The funders had no role in the design of the study, data collection, data analysis, interpretation of data, the decision to submit results for publication, or in the preparation, review, or approval of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Big data and glomerular disease: Uncovering common outcomes of rare disease,” on pages 2106–2108.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020111583/-/DCSupplemental.
Supplemental Document. Approach to determination of primary NS and presumed etiology.
Supplemental Table 1. Baseline characteristics among 907 adults with confirmed primary NS stratified by FSGS, NM, or MCD identified between 1996 and 2012.
Supplemental Table 2. Multivariable association of primary NS and subsequent ESKD with adjustment for time-updated comorbidities.
Supplemental Figure 1. Prioritized subgroups of adults with potential NS that underwent physician adjudication of medical records.
Supplemental Figure 2. Distribution of definite or presumed etiology in 1956 adults with confirmed NS.
Supplemental Figure 3. Kaplan–Meier survival curves for clinical outcomes in adults with primary NS versus matched controls.
References
- 1.Kodner C: Diagnosis and management of nephrotic syndrome in adults. Am Fam Physician 93: 479–485, 2016 [PubMed] [Google Scholar]
- 2.Hull RP, Goldsmith DJ: Nephrotic syndrome in adults. BMJ 336: 1185–1189, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orth SR, Ritz E: The nephrotic syndrome. N Engl J Med 338: 1202–1211, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Gipson DS, Selewski DT, Massengill SF, Modes MM, Desmond H, Lee L, et al.: NephCure accelerating cures institute: A multidisciplinary consortium to improve care for nephrotic syndrome. Kidney Int Rep 3: 439–446, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou YH, Lien YC, Hu FC, Lin WC, Kao CC, Lai CF, et al.: Clinical outcomes and predictors for ESRD and mortality in primary GN. Clin J Am Soc Nephrol 7: 1401–1408, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg AZ, Palmer M, Merlino L, Troost JP, Gasim A, Bagnasco S, et al.: The application of digital pathology to improve accuracy in glomerular enumeration in renal biopsies. PLoS One 11: e0156441, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gadegbeku CA, Gipson DS, Holzman LB, Ojo AO, Song PX, Barisoni L, et al.: Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int 83: 749–756, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hommos MS, Zeng C, Liu Z, Troost JP, Rosenberg AZ, Palmer M, et al.: Global glomerulosclerosis with nephrotic syndrome; the clinical importance of age adjustment. Kidney Int 93: 1175–1182, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Shaughnessy MM, Hogan SL, Poulton CJ, Falk RJ, Singh HK, Nickeleit V, et al.: Temporal and demographic trends in glomerular disease epidemiology in the Southeastern United States, 1986-2015. Clin J Am Soc Nephrol 12: 614–623, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chugh KS, Malik N, Uberoi HS, Gupta VK, Aggarwal ML, Singhal PC, et al.: Renal vein thrombosis in nephrotic syndrome: A prospective study and review. Postgrad Med J 57: 566–570, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llach F, Papper S, Massry SG: The clinical spectrum of renal vein thrombosis: Acute and chronic. Am J Med 69: 819–827, 1980 [DOI] [PubMed] [Google Scholar]
- 12.Wagoner RD, Stanson AW, Holley KE, Winter CS: Renal vein thrombosis in idiopathic membranous glomerulopathy and nephrotic syndrome: Incidence and significance. Kidney Int 23: 368–374, 1983. 10.1038/ki.1983.28 [DOI] [PubMed] [Google Scholar]
- 13.Haas M, Meehan SM, Karrison TG, Spargo BH: Changing etiologies of unexplained adult nephrotic syndrome: A comparison of renal biopsy findings from 1976-1979 and 1995-1997. Am J Kidney Dis 30: 621–631, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Gordon N, Lin T: The Kaiser Permanente Northern California adult member health survey. Perm J 20: 15–225, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, et al.: Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 76: 893–899, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Go AS, Magid DJ, Wells B, Sung SH, Cassidy-Bushrow AE, Greenlee RT, et al.: The Cardiovascular Research Network: A new paradigm for cardiovascular quality and outcomes research. Circ Cardiovasc Qual Outcomes 1: 138–147, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Ross TR, Ng D, Brown JS, Pardee R, Hornbrook MC, Hart G, et al.: The HMO Research Network Virtual Data Warehouse: A public data model to support collaboration. EGEMS (Wash DC) 2: 1049, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curb JD, Ford CE, Pressel S, Palmer M, Babcock C, Hawkins CM: Ascertainment of vital status through the National Death Index and the Social Security Administration. Am J Epidemiol 121: 754–766, 1985 [DOI] [PubMed] [Google Scholar]
- 19.Kitiyakara C, Eggers P, Kopp JB: Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis 44: 815–825, 2004 [PubMed] [Google Scholar]
- 20.Couser WG: Primary membranous nephropathy. Clin J Am Soc Nephrol 12: 983–997, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerlin BA, Ayoob R, Smoyer WE: Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol 7: 513–520, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glassock RJ: Prophylactic anticoagulation in nephrotic syndrome: A clinical conundrum. J Am Soc Nephrol 18: 2221–2225, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Barbour SJ, Greenwald A, Djurdjev O, Levin A, Hladunewich MA, Nachman PH, et al.: Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int 81: 190–195, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Kayali F, Najjar R, Aswad F, Matta F, Stein PD: Venous thromboembolism in patients hospitalized with nephrotic syndrome. Am J Med 121: 226–230, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Maas RJ, Deegens JK, Beukhof JR, Reichert LJ, Ten Dam MA, Beutler JJ, et al.: The clinical course of minimal change nephrotic syndrome with onset in adulthood or late adolescence: A case series. Am J Kidney Dis 69: 637–646, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Rankin AJ, McQuarrie EP, Fox JG, Geddes CC, MacKinnon B; Scottish Renal Biopsy Registry: Venous thromboembolism in primary nephrotic syndrome: Is the risk high enough to justify prophylactic anticoagulation? Nephron 135: 39–45, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Zhang LJ, Zhang Z, Li SJ, Meinel FG, Nance JW Jr, Zhou CS, et al.: Pulmonary embolism and renal vein thrombosis in patients with nephrotic syndrome: Prospective evaluation of prevalence and risk factors with CT. Radiology 273: 897–906, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Lee T, Derebail VK, Kshirsagar AV, Chung Y, Fine JP, Mahoney S, et al.: Patients with primary membranous nephropathy are at high risk of cardiovascular events. Kidney Int 89: 1111–1118, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ordoñez JD, Hiatt RA, Killebrew EJ, Fireman BH: The increased risk of coronary heart disease associated with nephrotic syndrome. Kidney Int 44: 638–642, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Mahmoodi BK, ten Kate MK, Waanders F, Veeger NJ, Brouwer JL, Vogt L, et al.: High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: results from a large retrospective cohort study. Circulation 117: 224–230, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Huang JA, Lin CH, Chang YT, Lee CT, Wu MJ: Nephrotic syndrome is associated with increased risk of ischemic stroke. J Stroke Cerebrovasc Dis 28: 104322, 2019 [DOI] [PubMed] [Google Scholar]
- 32.Singhal R, Brimble KS: Thromboembolic complications in the nephrotic syndrome: pathophysiology and clinical management. Thromb Res 118: 397–407, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Eneman B, Levtchenko E, van den Heuvel B, Van Geet C, Freson K: Platelet abnormalities in nephrotic syndrome. Pediatr Nephrol 31: 1267–1279, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Loscalzo J: Venous thrombosis in the nephrotic syndrome. N Engl J Med 368: 956–958, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Sun AZ, Shu YH, Harrison TN, Hever A, Jacobsen SJ, O’Shaughnessy MM, et al.: Identifying patients with rare disease using electronic health record data: The Kaiser Permanente Southern California membranous nephropathy cohort [published online ahead of print February 7, 2020]. Perm J 24: 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcelon N, Burgun A, Salomon R, Neuraz A: Electronic health records for the diagnosis of rare diseases. Kidney Int 97: 676–686, 2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.