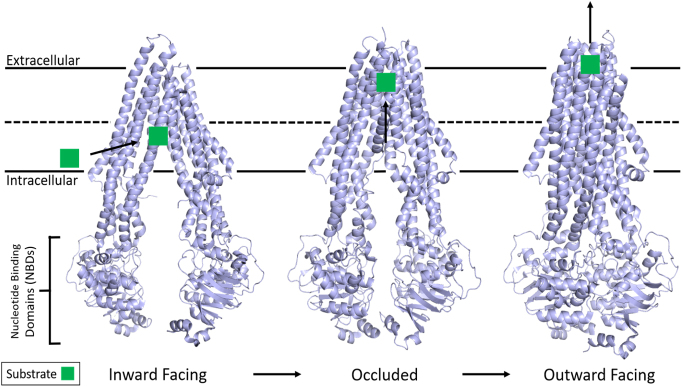
Figure 1.
Structure of P-gp and the basic mechanism of ATP hydrolysis and transport. P-gp undergoes large conformational changes in its transport mechanism. The membrane is delineated by solid black lines. Substrate from the inner leaflet first interacts with an inward-facing conformation of P-gp while two ATP molecules bind the free NBDs. The structure then adopts an occluded state with substrate bound at the apex of the TMDs while TM helices 4 and 10 significantly kink inwards, occluding the binding pocket. The outward-facing conformation is correlated with asymmetric ATP hydrolysis and solvent-exposed substrate diffusion into extracellular space before ADP release and the mechanism resetting. Adapted from PDB: 5KPI (inward)[94], 7A6C (occluded)[96], and 6C0V (outward)[95]. NBDs: Nucleotide-binding domains; TMDs: transmembrane domains.