Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can cause severe placental lesions leading rapidly to intrauterine fetal death (IUFD). From August 2020 to September 2021, in the pathology department of Toulouse Oncopole, we analyzed 50 placentas from COVID-19–positive unvaccinated mothers.
The purpose of our study is to describe the clinicopathological characteristics of these placental damages and to understand the pathophysiology.
Ten of them (20%) showed placental lesions with positive immunohistochemistry for SARS-CoV-2 in villous trophoblasts. In five cases (10%), we observed massive placental damage associating trophoblastic necrosis, fibrinous deposits, intervillositis, as well as extensive hemorrhagic changes due to SARS-CoV-2 infection probably responsible of IUFD by functional placental insufficiency. In five other cases, we found similar placental lesions but with a focal distribution that did not lead to IUFD but live birth.
These lesions are independent of maternal clinical severity of COVID-19 infection because they occur despite mild maternal symptoms and are therefore difficult to predict. In our cases, they occurred 1–3 weeks after positive SARS-CoV-2 maternal real-time polymerase chain reaction testing and were observed in the 2nd and 3rd trimesters of pregnancies. When these lesions are focal, they do not lead to IUFD and can be involved in intrauterine growth restriction.
Our findings, together with recent observations, suggest that future pregnancy guidance should include stricter pandemic precautions such as screening for a wider array of COVID-19 symptoms, enhanced ultrasound monitoring, as well as newborn medical surveillance.
Keywords: Intrauterine fetal death, SARS-Cov-2 infection, Pregnancy, COVID-19 placental lesions
1. Introduction
Vertical transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and pregnancy complications induction are serious concerns for pregnant individuals with COVID-19. Based on early RNA detection of SARS-CoV-2 after birth, vertical transmission's incidence of SARS-CoV-2 has been estimated to occur in around 2–3% of neonates [1,2]. The short- and long-term consequences of this infection on the newborn are still poorly understood. However, even if a neonate tests negative for SARS-CoV-2, different abnormal placental findings have been reported in cases of COVID-19–positive mothers [3]. Since the beginning of the pandemic, many nonspecific placental lesions have been described, including fetal and maternal vascular malperfusion, or chorioamnionitis, all negative in immunohistochemistry with the anti–SARS-CoV-2 antibody [4]. On the other hand, lesions characterized by trophoblastic necrosis, fibrinous deposits, and intervillositis are more suggestive of viral involvement and can lead to IUFD [5].
The purpose of our study is to describe the clinicopathological characteristics of placental damages associated with SARS-CoV-2 maternal infection in 50 placentas from COVID-19–positive unvaccinated mothers and to understand the pathophysiology. Histopathological findings are heterogeneous. Among positive SARS-CoV-2 placentas, specific histological findings are extremely variable in intensity from diffuse placental hemorrhagic lesions to focal perivillous fibrin deposition lesions possibly sequellar. In 10% of cases, lesions are diffuse and associated to an increase of IUFD in the 2nd and 3rd trimester of pregnancy.
2. Materials and methods
From August 2020 to September 2021, in the pathology department of Toulouse Oncopole (attached to the biggest maternity unit in Occitania [south of France] with more than 5000 births a year), we analyzed fifty placentas from COVID-19–positive unvaccinated mothers. Forty of them were normal or nonspecific at histological examination, whereas 10 of them showed particular abnormal histological signs reported here.
2.1. Immunohistochemistry
Automated classical immunohistochemical (IHC) stains were performed using the Benchmark ULTRA (Roche, Ventana Medical Systems, Innovation Park Drive Tucson, Arizona 85755, USA) on formalin-fixed and paraffin-embedded (FFPE) tissue sections (3 μm). After dewaxing, tissue slides were heat pretreated using a CC1 (pH8) buffer (05424569001, Roche) for 64 min at 98 °C. The slides were blocked for endogenous peroxidase activity and incubated with primary anti–2019-nCOV Spike rabbit polyclonal (A20022, ABclonal, Diagomics, Blagnac, France) (1/100 in Envision FLEX antibody diluent, K800621-2, Agilent, Santa Clara, CA 95051, USA) and anti–SARS-CoV-2 nucleocapsid mouse monoclonal (Ready to Use, clone BSB-134, BioSB, Diagomics, Blagnac, France) antibodies for 64 and 92 min, respectively. Targets were then visualized using the OptiView DAB detection kit (06396500001, Roche). The tissue slides were counterstained using hematoxylin (05277965001, Roche) for 8 min followed by postcoloration using Bluing reagent for 4 min at room temperature (05266769001, Roche). The slides were then dehydrated (ethanol and xylene) and mounted using xylene-based mounting (TissueTek Prisma®, Sakura Finetek Europe B.V., The Netherlands).
2.2. SARS-CoV-2 real-time polymerase chain reaction
SARS-CoV-2 genomic RNA was detected on the specimen taken by nasopharyngal swabs by Hologic Aptima SARs-CoV-2 transcription-mediated amplification on the Panther instrument as recommended by the manufacturer. For SARS-CoV-2 genomic RNA detection on placenta, samples were crushed and nucleic acids extracted using MagNA Pure 96 small volume protocol (Roche Diagnostics). The targets were localized on the RNA-dependent polymerase gene (IP2 and IP4, Institut Pasteur, Paris, France). Amplification and real-time detection were performed using qScript XLT 1-step RT-qPCR ToughMix (Quantabio, VWR International SAS, France) on Light Cycler 480 (Roche Diagnostics).
3. Results
In our study, 40 of the 50 placentas analyzed showed histological findings either normal (n = 16/40, 40%) or with various nonspecific lesions as previously described in the literature [4,6,7] such as fetal vascular malperfusion (n = 1/40) and maternal vascular malperfusion (n = 17/40, 42,5%), chorioamnionitis (n = 8/40, 20%), or focal intervillositis (n = 1/40) (Table 1 ) according to the Amsterdam Placental Consensus classification [8]. All these cases were negative with anti–SARS-CoV-2 antibody and real-time polymerase chain reaction (RT-PCR) in the placenta. Thirty-eight of them were associated with live births; the two other cases were late miscarriages, one associated with a monochorial biamniotic twin pregnancy with transfused transfusion syndrome and one in a context of septate uterus with a history of multiple miscarriages.
Table 1.
Pregnancy outcome and maternal and placental characteristics.
| Cases | Age of onset of maternal symptoms (WoG) | Term of delivery (WoG) | Time between maternal symptoms and delivery (days) | Pregnancy outcome | Maternal symptoms | Fetal weight (g) | Fetal weight (percentile) | Histological placental lesions |
|---|---|---|---|---|---|---|---|---|
| 1 | 32 | 34 + 1 | 15 | IUFD | Influenza-like illness + hyperthermia | 1640 | 3-5th | Diffuse TN + intervillositis + PFD + IH |
| 2 | 21 | 23 | 14 | IUFD | Influenza-like illness | 585 | <3rd | Diffuse TN + intervillositis + PFD + IH |
| 3 | 18 + 4 | 19 + 4 | 7 | IUFD | Influenza-like illness | 240 | <3rd | Diffuse TN + intervillositis + PFD + IH |
| 4 | 19 + 3 | 21 | 11 | IUFD | Hyperthermie | 380 | 10-25th | Diffuse TN + intervillositis + PFD + IH |
| 5 | 34 | 36 + 6 | 20 | IUFD | Influenza-like illness | 2990 | 50th | Diffuse TN + intervillositis + PFD + IH |
| 6 | unknown | 38 + 5 | unknown | Cesarean section for bradycardia and preeclampsia live newborn | Hyperthermia | 2440 | <5th | Focal TN + intervillositis + PFD |
| 7 | 30 | 33 | 21 | Cesarean section live newborn | Influenza-like illness | 1940 | 20-50th | Focal TN + intervillositis + PFD |
| 8 | 33 | 33 | 0 | Cesarean section live newborn | Influenza-like illness | 1730 | 30th | Focal TN + intervillositis + PFD |
| 9 | 35 | 37 + 3 | 18 | Cesarean section live newborn | Asymptomatic | 2465 | 10-25th | Focal TN + intervillositis + PFD |
| 10 | 35 + 4 | 40 + 4 | 35 | Vaginal delivery live newborn | Hyperthermia | 2860 | 5-10th | Focal TN + intervillositis + PFD |
| 11 | 32 | 39 | 49 | Vaginal delivery live newborn | Influenza-like illness | 2550 | 3-5th | MVM |
| 12 | 25 | 36 + 5 | 82 | Vaginal delivery live newborn | Hypoxemic pneumonia | 4250 | 95th | Normal |
| 13 | 31 | 32 | 7 | Cesarean section live newborn | Hypoxemic pneumonia | 1800 | 70th | Normal |
| 14 | 32 | 33 | 7 | Cesarean section live newborn | Influenza-like illness | 1700 | 30th | Normal |
| 15 | 31 + 3 | 37 | 45 | Cesarean section live newborn | Asymptomatic | 3100 | >75th | Normal |
| 16 | 30 | 30 + 5 | 5 | Cesarean section live newborn | Hypoxemic pneumonia | 1600 | >50th | MVM |
| 17 | 39 | 41 | 14 | Vaginal delivery live newborn | Asymptomatic | 4210 | >95th | MVM |
| 18 | 35 | 39 + 4 | 32 | Vaginal delivery live newborn | Asymptomatic | 3350 | >95th | Normal |
| 19 | 38 + 2 | 39 + 2 | 7 | Cesarean section live newborn | Unknown | 2890 | 25th | Normal |
| 20 | 29 + 2 | 30 | 5 | Cesarean section live newborn | Hypoxemic pneumonia | 1620 | >75th | Chorangiosis |
| 21 | 28 | 28 + 2 | 2 | Cesarean section live newborn | Hypoxemic pneumonia | 1100 | >50th | Chorangiosis |
| 22 | 37 | 39 + 1 | 15 | Vaginal delivery live newborn | Unknown | 2250 | <3rd | MVM |
| 23 | 35 | 38 + 1 | 22 | Vaginal delivery live newborn | Hypoxemic pneumonia | 3350 | >75th | Normal |
| 24 | 33 + 2 | 34 + 1 | 6 | Cesarean section live newborn | Hypoxemic pneumonia | 1935 | >25th | MVM |
| 25 | 13 | 40 + 6 | 195 | Vaginal delivery live newborn | Influenza-like illness | 2680 | <3rd | MVM |
| 26 | 34 + 2 | 41 + 5 | 46 | Vaginal delivery live newborn | Hypoxemic pneumonia | 3300 | 25th | Normal |
| 27 | 28 | 33 + 3 | 38 | Vaginal delivery live newborn | Unknown | 2130 | >75th | MVM |
| 28 | 25 | 40 + 4 | 109 | Vaginal delivery live newborn | Influenza-like illness | 3730 | 59th | Normal |
| 29 | 34 + 3 | 39 + 4 | 36 | Vaginal delivery live newborn | Unknown | 3120 | >25th | MVM |
| 30 | 26 | 38 + 2 | 86 | Vaginal delivery live newborn | Unknown | 2990 | 47th | Normal |
| 31 | 31 + 4 | 32 + 6 | 9 | Vaginal delivery live newborn | Hypoxemic pneumonia | 1505 | 25th | FVM |
| 32 | Unknown | 29 | Unknown | Vaginal delivery live newborn | Unknown | 1230 | >50th | Chorioamnionitis |
| 33 | 34 + 5 | 34 + 6 | 1 | Cesarean section live newborn | Asymptomatic | 2350 | >25th | MVM |
| 34 | 17 | 18 + 5 | 12 | Twins pregnancy: miscarriage | Unknown | 120 and 240 | 10th and >50th | Chorioamnionitis |
| 35 | 36 | 39 | 21 | Vaginal delivery live newborn | Asymptomatic | 3840 | >95th | Normal |
| 36 | 21 | 38 | 119 | Vaginal delivery live newborn | Influenza-like illness | 3715 | >95th | MVM |
| 37 | 32 | 39 | 49 | Vaginal delivery live newborn | Influenza-like illness | 3440 | >50th | Normal |
| 38 | 33 + 1 | 41 | 55 | Vaginal delivery live newborn | Unknown | 4200 | 90th | MVM + chorioamnionitis |
| 39 | 31 | 37 | 42 | Vaginal delivery live newborn | Unknown | 3390 | >75th | Normal |
| 40 | 4 | 41 | 259 | Vaginal delivery live newborn | Influenza-like illness | Unknown | x | Normal |
| 41 | 10 | 39 + 6 | 209 | Vaginal delivery live newborn | Unknown | 3090 | 20th | Focal intervillositis |
| 42 | 28 | 39 | 77 | Vaginal delivery live newborn | Influenza-like illness | 3470 | 47th | Chorioamnionitis |
| 43 | 4 | 39 | 245 | Vaginal delivery live newborn | Unknown | 3600 | 75th | Normal |
| 44 | 34 + 3 | 39 + 4 | 36 | Vaginal delivery live newborn | Unknown | 3120 | 25th | MVM |
| 45 | 18 + 5 | 28 + 5 | 70 | Vaginal delivery live newborn | Unknown | 1030 and 1220 | 25th and 50th | MVM |
| 46 | 34 | 40 + 4 | 46 | Vaginal delivery live newborn | Unknown | 3470 | 60th | Chorioamnionitis |
| 47 | 32 | 40 + 2 | 58 | Vaginal delivery live newborn | Unknown | Unknwon | X | MVM + chorioamnionitis |
| 48 | 13 | 23 + 3 | 73 | Miscarriage | 430 | NA | X | MVM + chorioamnionitis |
| 49 | 20 + 3 | 40 + 3 | 143 | Vaginal delivery live newborn | Unknown | 3260 | 40th | MVM + chorioamnionitis |
| 50 | 24 | 39 + 5 | 110 | Vaginal delivery live newborn | Unknown | 3260 | 25th | MVM |
Abbreviations: IUFD, intrauterine fetal death; TN, trophoblastic necrosis; PFD, perivillous fibrin deposition; IH, intervillous hemorrhage; MVM, maternal vascular malperfusion; FVM, fetal vascular malperfusion.
Among the 10 cases with significant placental lesions, five were associated with IUFD, whereas the other five were associated with live birth.
Five of the ten patients (Cases n°1–5) were hospitalized in our department for IUFD discovered between 19 weeks of gestation (WoG) and 36 + 6 WoG. None of them had a medical history. The pregnancy was marked by maternal COVID-19 infection between 18 + 4 WoG and 34 WoG, therefore between 1 and 3 weeks before IUFD occurred. Maternal symptoms were unspecific and mild, mainly hyperthermia (detailed in Table 1). Interestingly, three of them were tested for SARS-CoV-2 by nasopharyngeal swabs not because of symptoms suggestive of COVID but because they were considered contact cases. All five patients presented in our emergency unit for decreased fetal movements. Ultrasound examination discovered IUFD with a variable estimated fetal weight from <3rd percentile to 50th percentile. The standard IUFD examinations (CGH array, CMV, and Parvovirus B19 research in amniotic fluid, as well as maternal autoimmune tests excluding other causes of IUFD) were negative in the five cases. Placental bacteriology came back negative as well. All five placentas weighed within 10th-25th percentile for reference ranges. Cross-placental examination revealed unusual diffuse macroscopic lesions, characterized by several intraplacental and subchorial hematoma, dissociating the placenta tissue (Fig. 1, A3). These lesions involved at least 80% of the parenchyma.
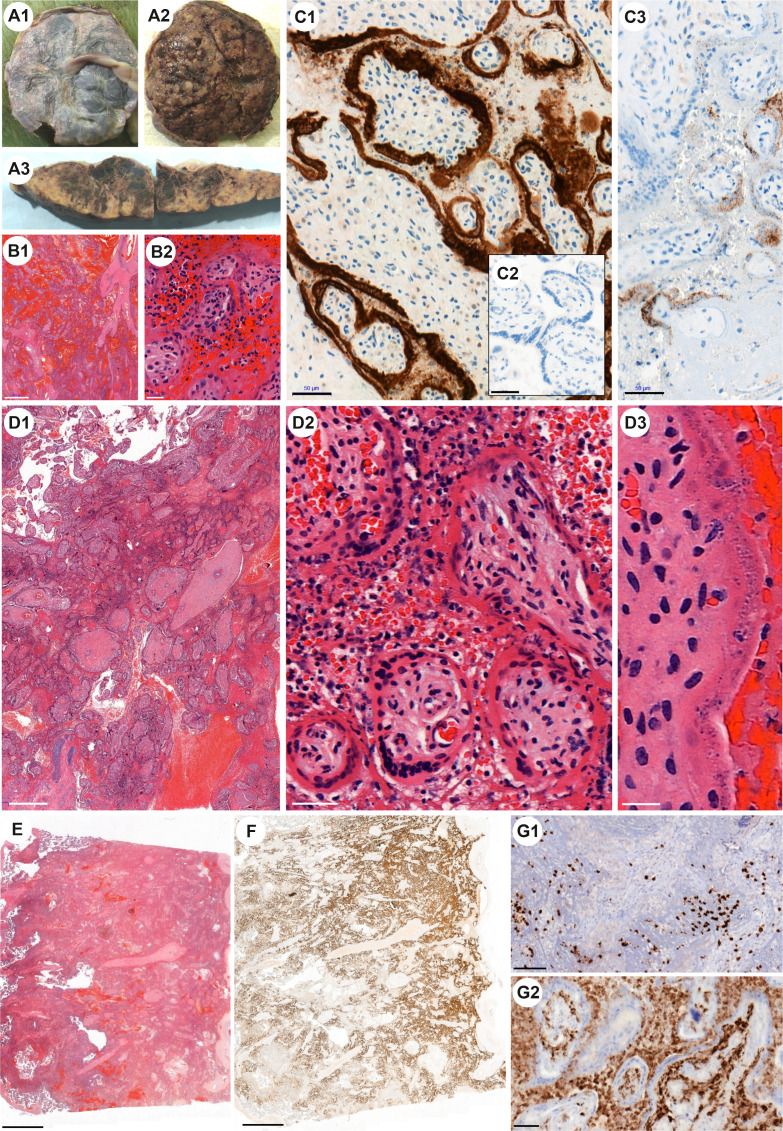
Fig. 1.
Case n°1: No significant macroscopic placental lesions on the chorionic A1 and basal A2 plates. A3 Unusual diffuse macroscopic lesions, characterized by several intraplacental and subchorial hematoma, dissociating the placental tissue. B1 Diffuse intervillous hemorrhagic lesions associated with extensive trophoblast necrosis and chronic intervillitis. Hematoxylin and eosin (H&E) staining. B2 Trophoblast necrosis and chronic intervillitis. H&E. C1 Brown staining at the periphery of the chorionic villi represents trophoblast infection identified by immunohistochemistry (IHC) using SARS-CoV-2 nucleocapsid protein antibody. Few inflammatory cells in the intervillous space show also positive staining. C2 Negative control (patient COVID + with no placental lesion), IHC with anti–SARS-CoV-2 nucleocapsid antibody show no positivity. C3 IHC with anti–SARS-CoV2-spike antibody show patchy granular cytoplasmic staining. Case n°2: Both D1 (H&E) and D2 (H&E) show massive hemorrhagic inundation of intervillous space and extensive villous trophoblast necrosis. Surrounding the villi, chronic histiocytic intervillositis and fibrin deposits are observed as well as several polynuclear. D3 (H&E) Villous trophoblast shows signs of cellular injury including karyorrhexis, fragmentation of trophoblast nuclei, and clearing of the cytoplasm. Case n°3: E Microscopic aspects comparable to cases 1 and 2 (H&E). F IHC with anti–SARS-CoV-2 nucleocapsid antibody is highly positive in necrosis trophoblast. Intervillous inflammatory infiltrate G1 with both T lymphocytes (IHC with CD3 antibody) and G2 monocytic cells (IHC with CD68 antibody).
Histologically, lesions were characterized by massive hemorrhagic inundation of intervillous space and extensive villous trophoblastic necrosis (Fig. 1, B1, D1-2, E). Surrounding the villi, chronic intervillositis and fibrin deposits were present (Fig. 1, B2, E2). Polymorphonuclears were observed, owing to hemorrhagic lesions. At high magnification, villous trophoblasts showed signs of cellular injury including karyorrhexis, fragmentation of trophoblast nuclei, and clearing of cytoplasm (Fig. 1, D3). Neither intravillous inflammation nor vascular thrombosis in villous capillaries was detected.
Viral immunostaining with SARS-CoV-2 antibodies (anti–SARS-CoV-2 nucleocapsid antibody and anti–SARS-CoV-2-spike antibody) was positive in syncytiotrophoblasts, cytotrophoblasts, but not endothelial cells (Fig. 1, C1-3, F). Intense positive cytoplasmic staining was observed in villous trophoblasts with anti–SARS-CoV-2 nucleocapsid (N) antibody (Fig. 1, C1, F). Immunostaining with anti–SARS-CoV-2-spike (S) antibody showed granular cytoplasmic staining (Fig. 1, C3). Detection of SARS-CoV-2 RNA in placenta confirmed these results with low cycle threshold (Ct < 20) in favor of high levels of genomic SARS-CoV-2 RNA.
Associated chronic intervillous inflammatory infiltrate was characterized by abundant presence of T lymphocytes (CD3 +, with no predominance of neither CD4+ nor CD8+ T-cell subsets) and CD68+ monocytic cells. Few mononuclear cells circulating in maternal vascular space also showed strong positivity with anti–SARS-CoV-2 nucleocapsid (N) antibody.
Among these five cases, two fetuses were autopsied and none had visceral malformation, nor visceral (lung, kidney, heart) positive SARS-CoV-2 immunostaining.
The five other cases (cases n°6–10) showed similar lesions associating trophoblastic necrosis, fibrinoid deposits, and intervillositis but focally located with less than 30% of the parenchyma involved (Fig. 2, A1). Immunostaining with anti–SARS-CoV-2 nucleocapsid (N) antibody showed positive cytoplasmic staining only located in pathological villous trophoblasts (Fig. 2, A2, A3). Likewise, the lymphohistiocytic infiltrate demonstrated by anti-CD3 and CD68 antibodies was limited to the lesion areas.
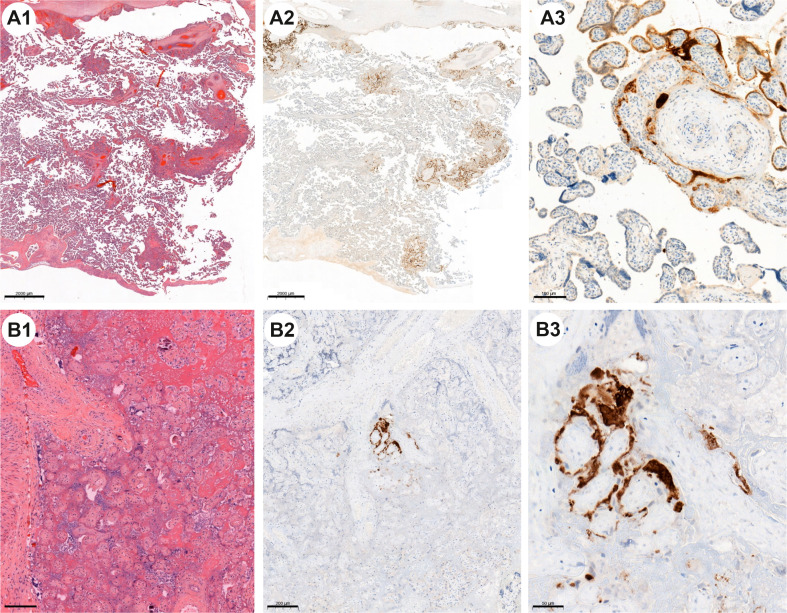
Fig. 2.
Case n°9: A1 (H&E) multifocal lesions occupying less than 30% of the placental parenchyma. A2 and A3 IHC with anti–SARS-CoV-2 nucleocapsid antibody is highly positive. Some areas show strong diffuse staining of the trophoblast (facing necrosis trophoblast) contrasting with negative nonaffected villi (facing preserved trophoblast). Case n°10: B1 (H&E) massive perivillous fibrin deposition with villous infarctions and without significant inflammatory infiltrate. B2 and B3 IHC with anti–SARS-CoV-2 nucleocapsid antibody. Note residual and focal immunostaining on this more fibrinous lesion.
One case (n°10) was particularly interesting because placental histological examination showed pathological foci characterized by perivillous fibrin deposition surrounding necrotic villi. These lesions were less inflammatory with more fibrin deposits, in the process of organization. Immunohistochemical anti–SARS-CoV-2 staining was not diffusely detected in trophoblasts as observed in other cases, but was only very focally present (Fig. 2, B1, B2, B3). Clinically, the time from maternal infection to childbirth was more than 2 months.
4. Discussion
4.1. Placenta's pathological lesions
In cases of maternal SARS-CoV-2 infection, most of the placental histopathological lesions largely reported were nonspecific such as fetal vascular malperfusion, maternal vascular malperfusion, chorangiosis, chorioamnionitis, and villositis [4,6,7]. Indeed 40 of the 50 placentas analyzed in our study (80%) showed histological findings either normal or with various nonspecific lesions as previously described in the literature, and all these cases were negative with anti–SARS-CoV-2 antibody and RT-PCR.
However, 10 of the 50 placentas analyzed showed specific placental lesions directly linked to SARS-CoV-2 infection because all were positive with anti–SARS-CoV-2 antibody and SARS-CoV-2 RT-PCR. Depending on their intensity, these lesions had variable consequences on pregnancy outcome ranging from intrauterine growth restriction (IUGR) to IUFD by functional placental insufficiency.
4.1.1. Placental damages linked to SARS-CoV-2 infection associated to IUFD
In five cases (cases n°1–5), placental damage was diffuse, involving at least 80% of parenchyma. Lesions were characterized by unusual massive hemorrhagic inundation of intervillous space and extensive villous trophoblastic necrosis associated with chronic intervillositis and fibrin deposits. Placental lesions observed in our cases were in agreement with the few IUFD cases described in literature but were additionally characterized by multiple intraplacental hemorrhagic lesions associated with extensive trophoblastic necrosis and chronic intervillositis [5,9,10]. The trophoblastic necrosis showed disappearance of trophoblastic cytoplasmic membranes, enlarged nuclei often vesicular with diffuse fine chromatin, or fragmentation of trophoblast nuclei possibly suggestive of viral toxicity of SARS-CoV-2 infection (Fig. 1, D3). This was supported by immunohistochemical studies showing positivity of villous trophoblast with SARS-CoV-2 antibodies. Otherwise, decidua arteriopathy was not seen but rather hemorrhagic changes dissociating the decidua, which is in agreement with literature [11]. Unlike other authors, we did not find fetal vascular thrombi [12].
The fact that these lesions were unusually intense and largely diffuse, both inflammatory and hemorrhagic, seems sufficient to explain functional placental insufficiency and its involvement in the fetal death due to placental destruction, especially because the IUFD assessment was negative.
On the clinical level, these lesions were observed between the 7th day and the 20th day after maternal infection occurred which is in agreement with the observation in the literature [11].
It is interesting to point out that hemorrhagic changes were especially marked in the diffuse placental damage associated to poor fetal outcome. These hematomas because of their intensity are unusual and cannot be explained by caesarian section.
Interestingly, Flores et al. analyzed the impact of COVID-19 infection on placental endothelium, by analyzing expression of von Willebrand factor (vWf), claudin-5, and vascular endothelial cadherin (VEC) in placentas of women with severe COVID-19 [13]. Their results showed an increase in the expression of vWf, suggesting the existence of a vascular thrombotic condition, and a decreased expression of both claudin-5 and VEC. Thus, enhanced vessel permeability is possibly responsible for a possible breach in the maternal–fetal interface leading to hemorrhages in the decidua and the intervillous space.
In the setting of adults SARS-CoV-2 infections, hemorrhagic lesions affecting other different organs have also been described such as hemorrhagic colitis [14], hemorrhagic pericardial effusion with tamponade [15], and hemorrhagic encephalopathy related to blood–brain barrier breakdown [16,17]. Some authors have suggested that these lesions are the result of a cytokine storm syndrome responsible for widespread microvascular injury [18].
4.1.2. Focal lesions linked to SARS-CoV-2 infection
In five other cases (cases n°6–10), lesions were focal or plurifocal mainly associated with IUGR and involved less than 30% of the parenchyma. On microscopic examination, several focal lesions were observed, still characterized by trophoblastic necrosis, chronic histiocytic intervillositis, and perivillous fibrin deposition, contrasting with nonlesional areas. Hemorrhagic changes were absent or rare.
Immunohistochemistry with anti–SARS-CoV-2 antibody was in agreement with the histological aspect and showed diffuse trophoblastic positivity in the lesion foci, whereas no positivity was noted elsewhere.
Interestingly, in case 10, the time from maternal infection to childbirth was more than 2 months, which is much longer than the 3 weeks observed in IUFD cases. This could suggest that lesions induced by SARS-CoV-2 infection evolve over time with only focal or even negative immunostaining for SARS-CoV-2, making the histological diagnosis of SARS-CoV-2 infection difficult.
4.2. SARS-CoV-2 vertical transmission
Among placentas and neonates tested for SARS-CoV-2 infection, a minority of neonates (2–3%) and placental samples (around 20%) tested positive for SARS-CoV-2 infection from the infected mother. This is what we have as well in our study because 10 of the 50 placentas tested positive for SARS-CoV-2 (20%) [19].
On reminder, SARS-CoV-2 infects tissues via its receptor angiotensin-converting enzyme 2 (ACE2), and its entry into cells requires spike protein cleavage by the serine protease TMPRSS2. ACE2 is highly expressed in maternal–fetal interface cells, such as syncytiotrophoblasts and cytotrophoblasts from 7 weeks onward and across gestation with similar intensity and distribution of ACE2 staining [20,21]. It is believed that high cellular coexpression of ACE2 (allowing virus attachment in cells) and TMPRSS2 (allowing virus penetration in cells) is required for viral transmission [22]. However, this rare coexpression in placenta could explain the high heterogeneity of placental lesions and thus variable and low occurrence of SARS-CoV-2 transplacental infection [13], as we expose it in case n°10 where lesions were highly heterogeneous (Fig. 1, D).
Schwartz et al. summarized the spectrum of pathology findings from pregnant women with COVID-19 based on the infection status of their infants. Authors concluded that co-occurrence of chronic histiocytic intervillositis and trophoblastic necrosis appears to be a risk factor for placental infection with SARS-CoV-2 as well as for maternal–fetal viral transmission and suggest a potential mechanism by which the coronavirus can breach the maternal–fetal interface [5]. However, the intensity and acute onset of the placental lesions could explain the rare positive fetal cases described to date because the death occurs rapidly by placental functional insufficiency before a possible fetal viral diffusion.
4.3. Correlation between severity of maternal signs and placental damage
In our study, placental lesions did not appear to be correlated with maternal infection severity. Indeed, third trimester's pregnant women placentas received in our laboratory showed subnormal placental examination while they presented severe symptoms with respiratory distress requiring emergency fetal extraction. On the other hand, second trimester's or third trimester's maternal infections have been reported several times with IUFD despite mild maternal COVID-19 symptoms [12,[23], [24], [25], [26]]. This makes clinical monitoring difficult and raises the question of ultrasound monitoring. Retrospectively, we did not find any ultrasound finding in the five cases with IUFD and suggest a possible rapid and fulminant onset of placental damage within a period of 1–3 weeks.
5. Conclusion
SARS-CoV-2 infection can cause severe placental lesions associating chronic inflammation with trophoblastic necrosis and massive hemorrhage leading rapidly to placental destruction and IUFD as observed in our laboratory. These lesions are independent of maternal symptoms because they occur despite mild maternal symptoms and are therefore difficult to predict. In our cases, they occurred 1–3 weeks after positive SARS-CoV-2 maternal RT-PCR testing and were observed in the 2nd and 3rd trimesters of pregnancy. IUFD associated to SARS-CoV-2 placental lesions is not so rare because in our study they occurred in 5 of 50 cases (10%). These lesions can also appear focally in the placenta and lead to live birth with sometimes IUGR. The discovery of such lesions in the placenta should initiate a medical monitoring of the newborns for which the long-term effects are not known to date. Moreover, focal SARS-CoV-2 lesions seem to evolve over time with more fibrin deposits, becoming difficult to diagnose because SARS-CoV-2 immunostaining could become negative. This study highlights the need for systematic placental and fetal gross and microscopic evaluation, which can help elucidate the pathophysiology of COVID-19.
Acknowledgments
We thank the regional agency of Occitania for its financial support dedicated to the virological analyses in pregnant women. Contributions: C. D. wrote the manuscript with support from M. G., V. J., S. F., D. D., J. R., and O. J. M. C. S. and J. A. helped supervise the project. M. G., A. S., L. B., and C. V. followed the clinical cases. C. P. did the viral experiments. N. V. A. organized the figure and did the immunohistochemistry perfection.
Footnotes
Disclosures: None.
References
- 1.Kotlyar A.M., Grechukhina O., Chen A., Popkhadze S., Grimshaw A., Tal O., et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:35–53 e33. doi: 10.1016/j.ajog.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamouroux A., Attie-Bitach T., Martinovic J., Leruez-Ville M., Ville Y. Evidence for and against vertical transmission for severe acute respiratory syndrome coronavirus 2. Am J Obstet Gynecol. 2020;223:91 e91–91 e94. doi: 10.1016/j.ajog.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong Y.P., Khong T.Y., Tan G.C. The effects of COVID-19 on placenta and pregnancy: what do we know so far? Diagnostics (Basel) 2021;11 doi: 10.3390/diagnostics11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hecht J.L., Quade B., Deshpande V., Mino-Kenudson M., Ting D.T., Desai N., et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod Pathol. 2020;33:2092–2103. doi: 10.1038/s41379-020-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz D.A., Morotti D. Placental pathology of COVID-19 with and without fetal and neonatal infection: trophoblast necrosis and chronic histiocytic intervillositis as risk factors for transplacental transmission of SARS-CoV-2. Viruses. 2020;12 doi: 10.3390/v12111308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baergen R.N., Heller D.S. Placental pathology in covid-19 positive mothers: preliminary findings. Pediatr Dev Pathol. 2020;23:177–180. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khong T.Y., Mooney E.E., Ariel I., Balmus N.C., Boyd T.K., Brundler M.A., et al. Sampling and definitions of placental lesions: Amsterdam placental workshop group Consensus statement. Arch Pathol Lab Med. 2016;140:698–713. doi: 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- 9.Bertero L., Borella F., Botta G., Carosso A., Cosma S., Bovetti M., et al. Placenta histopathology in SARS-CoV-2 infection: analysis of a consecutive series and comparison with control cohorts. Virchows Arch. 2021;479:715–728. doi: 10.1007/s00428-021-03097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosier H., Farhadian S.F., Morotti R.A., Deshmukh U., Lu-Culligan A., Campbell K.H., et al. SARS-CoV-2 infection of the placenta. J Clin Invest. 2020;130:4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouachba A., Allias F., Nadaud B., Massardier J., Mekki Y., Bouscambert Duchamp M., et al. Placental lesions and SARS-Cov-2 infection: diffuse placenta damage associated to poor fetal outcome. Placenta. 2021;112:97–104. doi: 10.1016/j.placenta.2021.07.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gioia C.D., Zullo F., Vecchio R.C.B., Pajno C., Perrone G., Galoppi P., et al. Stillbirth and fetal capillary infection by SARS-CoV-2. Am J Obstet Gynecol MFM. 2021:100523. doi: 10.1016/j.ajogmf.2021.100523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flores-Pliego A., Miranda J., Vega-Torreblanca S., Valdespino-Vazquez Y., Helguera-Repetto C., Espejel-Nunez A., et al. Molecular insights into the thrombotic and microvascular injury in placental endothelium of women with mild or severe COVID-19. Cells. 2021;10:364. doi: 10.3390/cells10020364. 10. Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvalho A., Alqusairi R., Adams A., Paul M., Kothari N., Peters S., et al. SARS-CoV-2 gastrointestinal infection causing hemorrhagic colitis: implications for detection and transmission of COVID-19 disease. Am J Gastroenterol. 2020;115:942–946. doi: 10.14309/ajg.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster B., Liaqat A., Chib A., Bolton S.S., Kendig A.C. An unusual presentation of COVID-19: hemorrhagic pericardial effusion with tamponade physiology. Cureus. 2021;13:e13438. doi: 10.7759/cureus.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krett J.D., Jewett G.A.E., Elton-Lacasse C., Fonseca K., Hahn C., Au S., et al. Hemorrhagic encephalopathy associated with COVID-19. J Neuroimmunol. 2020;346:577326. doi: 10.1016/j.jneuroim.2020.577326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y., Xia F., Li Y., Li H., Ma L., Hu X., et al. Changes in characteristics, treatment and outcome in patients with hemorrhagic stroke during COVID-19. J Stroke Cerebrovasc Dis. 2021;30:105536. doi: 10.1016/j.jstrokecerebrovasdis.2020.105536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. Hlh across Speciality Collaboration UK: COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharps M.C., Hayes D.J.L., Lee S., Zou Z., Brady C.A., Almoghrabi Y., et al. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta. 2020;101:13–29. doi: 10.1016/j.placenta.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui D., Liu Y., Jiang X., Ding C., Poon L.C., Wang H., et al. Single-cell RNA expression profiling of SARS-CoV-2-related ACE2 and TMPRSS2 in human trophectoderm and placenta. Ultrasound Obstet Gynecol. 2021;57:248–256. doi: 10.1002/uog.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gengler C., Dubruc E., Favre G., Greub G., de Leval L., Baud D. SARS-CoV-2 ACE-receptor detection in the placenta throughout pregnancy. Clin Microbiol Infect. 2021;27:489–490. doi: 10.1016/j.cmi.2020.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pique-Regi R., Romero R., Tarca A.L., Luca F., Xu Y., Alazizi A., et al. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? Elife. 2020;9 doi: 10.7554/eLife.58716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marinho P.S., da Cunha A., Chimelli L., Avvad-Portari E., Andreiuolo F.D.M., de Oliveira-Szejnfeld P.S., et al. Case report: SARS-CoV-2 mother-to-child transmission and fetal death associated with severe placental thromboembolism. Front Med (Lausanne) 2021;8:677001. doi: 10.3389/fmed.2021.677001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poisson T.M., Pierone G., Jr. Placental pathology and fetal demise at 35 weeks of gestation in a woman with SARS-CoV-2 infection: a case report. Case Rep Womens Health. 2021;30 doi: 10.1016/j.crwh.2021.e00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popescu D.E., Cioca A., Muresan C., Navolan D., Gui A., Pop O., et al. A case of COVID-19 pregnancy complicated with hydrops fetalis and intrauterine death. Medicina (Kaunas) 2021;57:667. doi: 10.3390/medicina57070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richtmann R., Torloni M.R., Oyamada Otani A.R., Levi J.E., Crema Tobara M., de Almeida Silva C., et al. Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: a case series. Case Rep Womens Health. 2020;27 doi: 10.1016/j.crwh.2020.e00243. [DOI] [PMC free article] [PubMed] [Google Scholar]