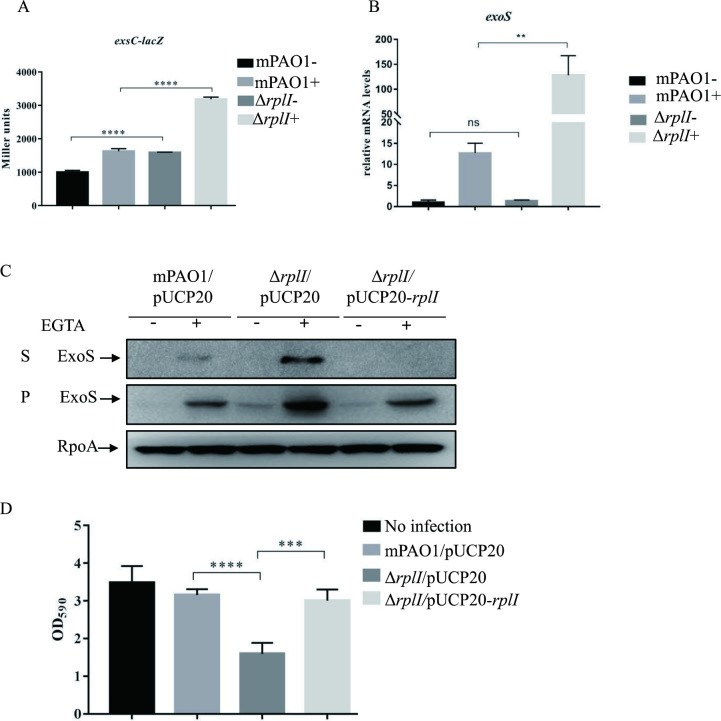
Fig 1. RplI represses T3SS expression and cytotoxicity.
(A) mPAO1 and ΔrplI containing the PexsC-lacZ transcriptional fusion were grown to an OD600 of 1.0 in LB with (+) or without (-) 5 mM EGTA and subjected to β-galactosidase assays. Each assay was performed in triplicate, and the error bars indicate standard deviations. ****P < 0.0001 compared with wild-type mPAO1 by Student’s t test. (B) Relative exoS mRNA levels in the mPAO1 and ΔrplI strains. Total RNA was isolated under T3SS inducing (+) and non-inducing (-) conditions with 5 mM EGTA, and the exoS mRNA levels were determined by real-time qPCR using rpsL as the internal control. ns, not significant, **P < 0.01, by Student’s t test. (C) Expression and secretion of ExoS in mPAO1/pUCP20, ΔrplI/pUCP20 and ΔrplI/pUCP20-rplI. Bacteria were cultured to an OD600 of 1.0 in LB medium with or without 5 mM EGTA. Proteins in supernatants (S) and pellets (P) from equivalent bacterial cells were loaded onto 12% SDS-PAGE gels and probed with an antibody against ExoS or an anti-RpoA antibody. The data shown represent the results from three independent experiments. (D) Cytotoxicity of mPAO1/pUCP20, ΔrplI/pUCP20 and ΔrplI/pUCP20-rplI. HeLa cells were infected with the indicated strains at an MOI of 50. Three hours post infection, cells attached to the 24-well plate were washed with PBS and stained with crystal violet. The cell-associated crystal violet was dissolved in ethanol and quantified by measuring the OD590. HeLa cells with no bacterial infection served as a control. ***P< 0.001, ****P < 0.0001 by Student’s t test.