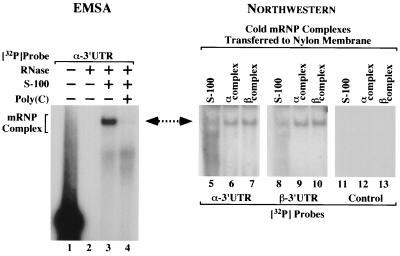
FIG. 4.
Fully assembled α and β complexes exchange α- and β-globin 3′ UTRs. In vitro-transcribed α- and β-globin 3′ UTRs were incubated in MEL cell S-100 extract and then resolved on a single polyacrylamide gel. (Left) A reaction utilizing 32P-labeled α 3′ UTR indicates the migration of the α complex (lane 3). Undigested and fully digested probes, as well as a homodeoxyribopoly(C)-competed reaction, were included as controls (lanes 1, 2, and 4). The composition of each reaction is indicated above the autoradiograph, and the position of the α complex is shown on the left. (Right) On the same gel, reactions utilizing α 3′ UTRs (lanes 6, 9, and 12) and β 3′ UTRs (lanes 7, 10, and 13) were resolved in triplicate. Control lanes 5, 8, and 11 contained S-100 extract alone. The complexes were transferred to a nylon membrane and were subsequently probed with either 32P-labeled α 3′ UTR, 32P-labeled β 3′ UTR, or an unrelated [32P]mRNA control (indicated at bottom), and autoradiographs were exposed. The composition of each reaction is indicated above the autoradiograph. A double-headed arrow emphasizes comigration of the α complex and the membrane-bound mRNP complexes that exchange 32P-labeled α and β 3′ UTRs.