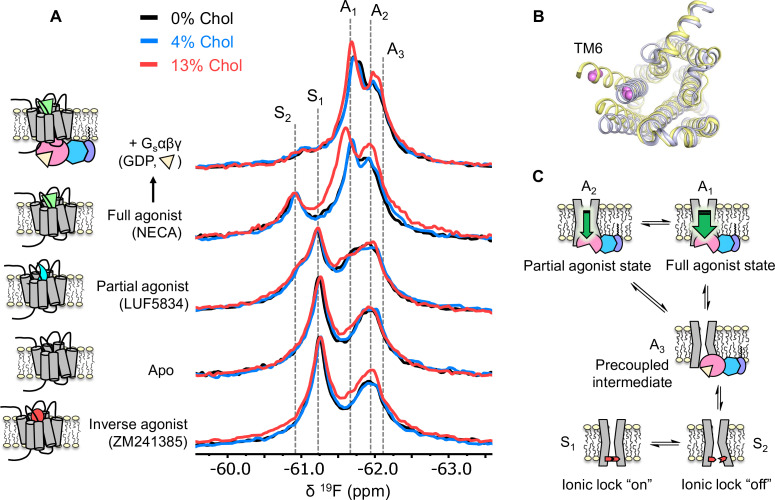
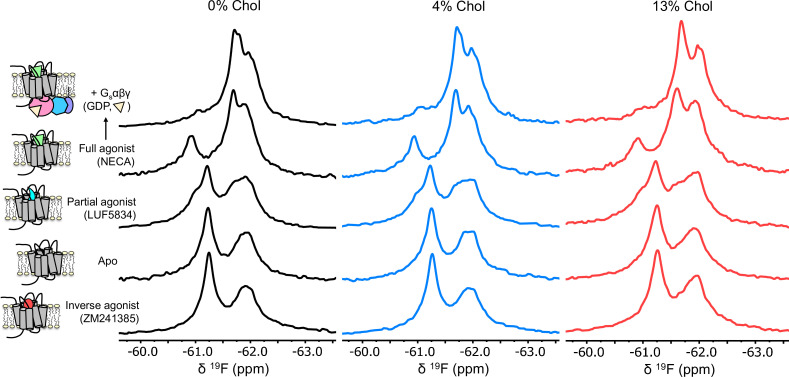
Figure 3. Cholesterol induces a small population increase in the active state conformers of A2AR.
(A) 19F NMR spectra of A2AR in nanodiscs containing 0, 4, and 13% cholesterol, as a function of ligand and G protein. Two inactive states (S1-2) and three active states (A1-3), previously identified, are indicated by gray dashed lines. For each ligand condition, spectra from the three cholesterol concentrations are normalized via their inactive state intensity. (B) Intracellular view of an inactive (gray, PDB: 4EIY) and an active (yellow, PDB: 5G53) crystal structure of A2AR highlighting the movement of TM6 upon activation. The 19F-labeling site is shown in violet. (C) Cartoon representations of the key functional states of A2AR indicated in (A). At the bottom are two inactive states (S1 and S2) where a conserved salt bridge is either intact or broken. A3 is an intermediate state that facilitates G-protein recognition and precoupling. A1 and A2 are active states that drive nucleotide exchange. While A1 is more efficacious and preferentially stabilized by the full agonist, A2 is less efficacious and reinforced by a partial agonist.