Fig. 2.
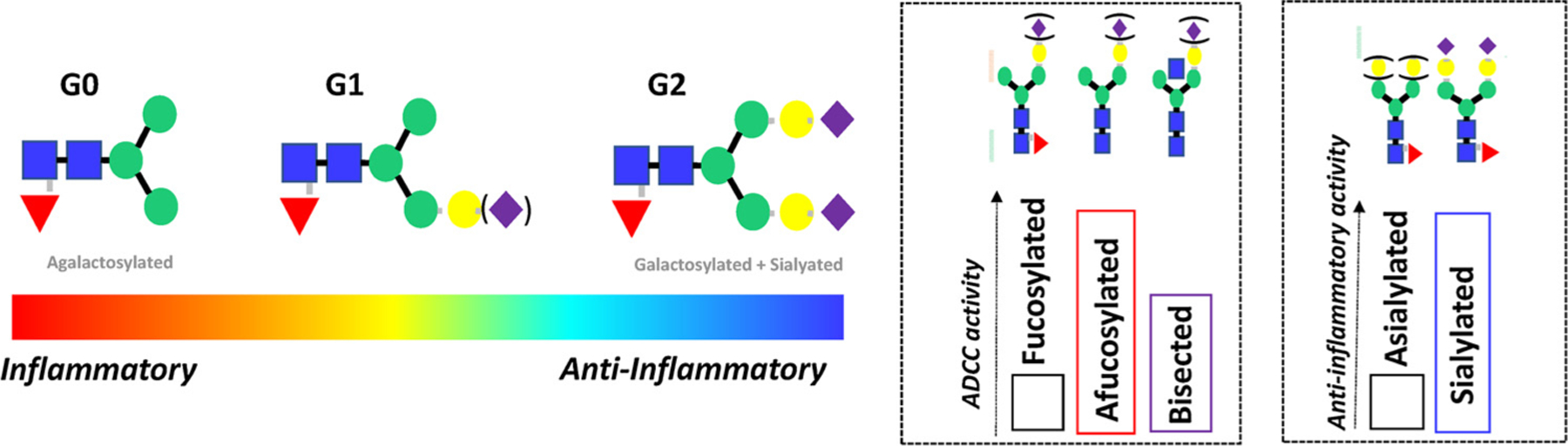
The functional consequences in antibody glycan changes. Changes in galactosylation have been observed across a multitude of human diseases, linked to alterations in antibody inflammatory activity. Specifically, an accumulation of agalactosylated antibodies observed across inflammatory diseases (auto-immunity, infections, malignancy), whereas increased galactosylation is associated with less-inflammatory conditions (pregnancy). Moreover, specific glycan changes have been exploited in the monoclonal therapeutics field demonstrating the critical role of glycans in shaping antibody effector function. For example, removal of fucose reproducibly enhances ADCC activity, whereas changes in bisecting GlcNAc alone have a more modest effect. Additionally, sialylation reproducibly enhance the anti-inflammatory activity of IVIG in vivo.
