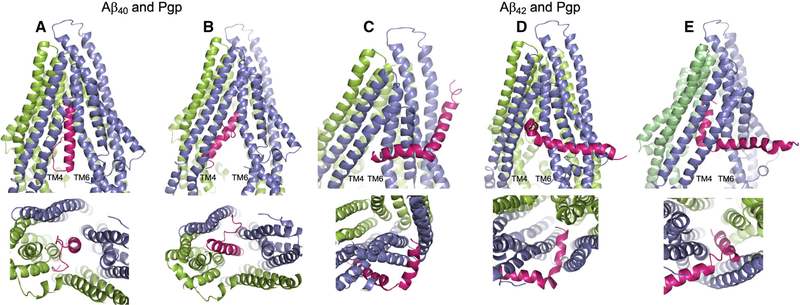
Fig. 2.
Docking of the Aβ40 and Aβ42 peptides to Pgp. The server CLUSPRO 2.0 was used to dock amyloid peptides Aβ40 (PDB: 1BA4) and Aβ42 (PDB: 1iYT) to Pgp (PDB: 4m1m). (A–E) Are side (upper) and bottom (lower) views of either peptide (hot pink) docked to Pgp (slate blue and chartreuse green). Pgp is represented with the domain swapped TM helices 4 and 5 (blue) shown at the left of each upper panel; and the opposite domain swapped TMs 10 and 11 (green) at the back right of each side view panel. (A) Aβ40 docked to Pgp with the nonpolar, helical end of the peptide uppermost in the Pgp cavity; and (B) Aβ40 docked to Pgp with the polar, random coil end of the peptide uppermost in the Pgp cavity. (C–E) Aβ42 docked to Pgp with the peptide at different stages of docking with Pgp. In each of the three docked modes, Aβ42 engages Pgp in the same orientation. (C) The short, nonpolar, C-terminal helix at the Pgp ‘gate’ between TM4 and TM6; (D) the Aβ42 helix within the Pgp cavity and at right angles to the Pgp TM helices; and (E) the helix wholly within the Pgp cavity and parallel to the Pgp TM helices. The figure was rendered with PYMOL (Molecular Graphics System, v2.4.0 Schrodinger). Standard settings were used, and no additional features such as attraction or repulsion were used. Scores for all docking modes were almost identical.