Abstract
The Kv1.3 channel has been widely demonstrated to play crucial roles in the activation and proliferation of T cells, which suggests that selective blockers could serve as potential therapeutics for autoimmune diseases mediated by T cells. We previously described that the toxin mimic FS48 from salivary gland of Xenopsylla cheopis downregulates the secretion of proinflammatory factors by Raw 264.7 cells by blocking the Kv1.3 channel and the subsequent inactivation of the proinflammatory MAPK/NF-κB pathways. However, the effects of FS48 on human T cells and autoimmune diseases are unclear. Here, we described its immunomodulatory effects on human T cells derived from suppression of Kv1.3 channel. Kv1.3 currents in Jurkat T cells were recorded by whole-cell patch-clamp, and Ca2+ influx, cell proliferation, and TNF-α and IL-2 secretion were measured using Fluo-4, CCK-8, and ELISA assays, respectively. The in vivo immunosuppressive activity of FS48 was evaluated with a rat DTH model. We found that FS48 reduced Kv1.3 currents in Jurkat T cells in a concentration-dependent manner with an IC50 value of about 1.42 μM. FS48 also significantly suppressed Kv1.3 protein expression, Ca2+ influx, MAPK/NF-κB/NFATc1 pathway activation, and TNF-α and IL-2 production in activated Jurkat T cells. Finally, we show that FS48 relieved the DTH response in rats. We therefore conclude that FS48 can block the Kv1.3 channel and inhibit human T cell activation, which most likely contributes to its immunomodulatory actions and highlights the great potential of this evolutionary-guided peptide as a drug template in future studies.
Keywords: Xenopsylla cheopis, FS48, potassium channel, Jurkat T cells, immunomodulator, autoimmune disease
Abbreviations: DTH, delayed-type hypersensitivity; NFATc1, nuclear factor of activate T cells c1; qRT-PCR, quantitative real-time PCR; TEM, effector memory T cell
Kv1.3 channel belongs to the Shaker group of potassium channels, which is highly expressed in effector memory T (TEM) cells and makes up the dominant K+ conductance for these cells (1). Kv1.3 channel in company with KCa channel (Ca2+ activated-potassium) mediates the cell resting membrane potential and provides the electrochemical driving force for Ca2+ influx through the CRAC (Ca2+ release-activated Ca2+) channel. Increased intracellular Ca2+ activated the nuclear factor of activate T cells c1 (NFATc1) and nuclear factor NF-κB, which mediate the activation, proliferation, and the secretion of cytokines of T cells, such as TNF-α and IL-2 (2, 3). Many studies have proved that blockage of Kv1.3 channel causes the inhibition of Ca2+ influx and the production of proinflammatory cytokines, thus exhibiting anti-inflammatory and immunomodulatory effects (4, 5, 6, 7, 8, 9). In many autoimmune animal models such as delayed-type hypersensitivity (DTH), type I diabetes, rheumatoid arthritis, chronic asthma, multiple sclerosis, psoriasis and diseases associated TEM cells are reported to remarkably increase Kv1.3 channel expression, migrate into tissues, release inflammatory cytokines, and cause detrimental inflammatory reaction after activation (8, 9, 10). The proliferation and cytokine secretion of these cells can be suppressed by Kv1.3 inhibitors (1, 10, 11, 12, 13, 14). Consequently, Kv1.3 has been regarded as a novel therapeutic target for many T-cell-mediated autoimmune diseases (8, 10). Hence, the discovery and development of specific Kv1.3 inhibitors have been widely carried out to prepare novel immunomodulators and immunosuppressants.
Natural peptide toxins are gradually acknowledged as potential therapeutic option for many diseases, which is attributed to their antitumor, immunomodulation, analgesic, and antimicrobial properties (8, 15). We previously identified and characterized FS48, a novel potassium channel inhibitor from the salivary gland of Xenopsylla cheopis (16). FS48 downregulates the secretion of proinflammatory NO, IL-1β, IL-6, and TNF-α by Raw 264.7 cells via Kv1.3 channel blockage and expression suppression as well as MAPK/NF-κB pathway inactivation. The present study aimed to evaluate the blocking effect of FS48 on Kv1.3 channel in human T cells and to clear its immunomodulatory mechanism. We first determined the effect of FS48 on the Kv1.3 channels and Ca2+ influx as well as its downstream signaling pathways. Moreover, we examined the effect of FS48 on the production of TNF-α and IL-2 with Kv1.3-siRNA. Finally, a DTH model was used to identify the immunomodulatory effect of FS48 in vivo. Our results show for the first time that FS48 inhibits Kv1.3-mediated currents, decreases Ca2+ influx and Ca2+-activated transcriptional factors, correspondingly suppressing the activation, proliferation, the release of IL-2 and TNF-α of Jurkat T cells. Therefore, we may conclude that FS48 can act in T cells and play an immunomodulatory role through Kv1.3 channel.
Results
FS48 blocked Kv1.3 channel currents in Jurkat T cells
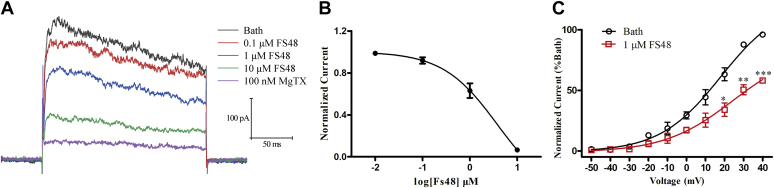
We performed patch-clamp electrophysiological recording assay in the whole-cell mode to evaluate the effect of FS48 on Kv1.3 currents in Jurkat T cells. As shown in Figure 1A, at test potentials positive to +40 mV, the blocking effects of FS48 at the concentrations from 0.1 to 10 μM on Kv1.3 channels were demonstrated to be concentration-dependent and the highest concentration of FS48 decreased the Kv1.3 currents by 75.42% ± 7.48% (n = 5) (Fig. 1A). Meanwhile, 100 nM MgTx significantly reduced the current by 93.75% ± 3.58% (n = 5). The Hill equation analysis showed the IC50 value of FS48 on Kv1.3 peak current was calculated to be 1.42 ± 0.12 μM (Fig. 1B). Moreover, the current–voltage curve indicated that FS48 didn’t affect the property and the activation of Kv1.3 channels (Fig. 1C), which was the same as our previous observed in cloned human Kv1.3 channels of HEK-293T and Raw 264.7 cells.
Figure 1.
Modulation of FS48 on endogenous voltage-gated potassium channels.A, representative traces of MgTx and different concentrations of FS48 suppressing the Kv1.3 currents in Jurkat T cells. Currents were elicited by applying 200 ms depolarization pulses from a holding potential of −70 mV to +40 mV in Jurkat T cells. B, concentration–response curve of FS48 inhibiting Kv1.3 currents in Jurkat T cells. Currents were normalized to the control and fitted by a Hill equation. C, current–voltage relationships (I-V). Test potentials were ranged from −50 mV to +40 mV with 10 mV increment steps. Y-axis represents the currents at different activation potential and normalized to the bath current at +40 mV in the present (red) or absent (black) of FS48; The solid lines represent the average Boltzmann sigmoidal fits. Data are shown as mean ± SD (n ≥ 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 compared with bath group.
FS48 inhibited Kv1.3 protein expression
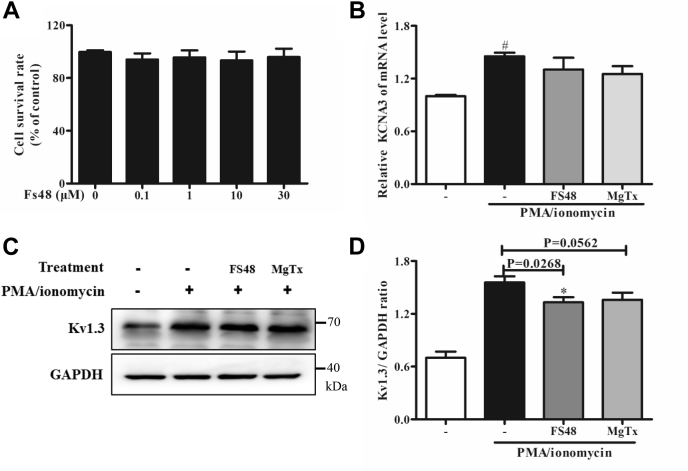
Cell viability measurements showed that FS48 at the concentrations from 0 to 30 μM for up to 24 h didn’t inhibit or promote Jurkat T cells survival (Fig. 2A). In order to identify whether FS48 can inhibit Kv1.3 protein expression, Jurkat T cells were incubated with FS48 and MgTx for 8 h and 24 h before quantitative real-time PCR (qRT-PCR) and Western blot analysis, respectively. As shown in Figure 2, B–D, 3 μM FS48 did not modify Kv1.3 mRNA but reduced the amount of Kv1.3 channel protein by 24.67% ± 5.64%. However, 24 h pretreatment with MgTX (50, 100, and 200 nM) had no obvious effects on Kv1.3 channel protein expression (p > 0.05) (Figs. 2, C and D and S2).
Figure 2.
Effects of FS48 on mRNA and protein expression of Kv1.3 channel.A, the viability of Jurkat T cells incubated with indicated concentrations of FS48 for 24 h. B, the relative expression analysis of KCNA3 mRNA in the presence and absence of FS48 and MgTx by qRT-PCR. C, Kv1.3 protein expression analysis of Kv1.3 channel. The cells were treated with PMA/ionomycin (50 ng/ml; 1 μg/ml) for 24 h after incubated with 3 μM FS48 and 100 nM MgTx for 1 h and then were collected for Western blot analysis. D, the ratios of Kv1.3 proteins to GAPDH. Quantity One software (Bio-Rad) was used for band density analysis. Data are shown as mean ± SD (n ≥ 3). #p < 0.05, compared with negative control group. ∗p < 0.05, compared with model group.
FS48 suppressed the Ca2+ influx to Ca2+-depleted Jurkat T cells
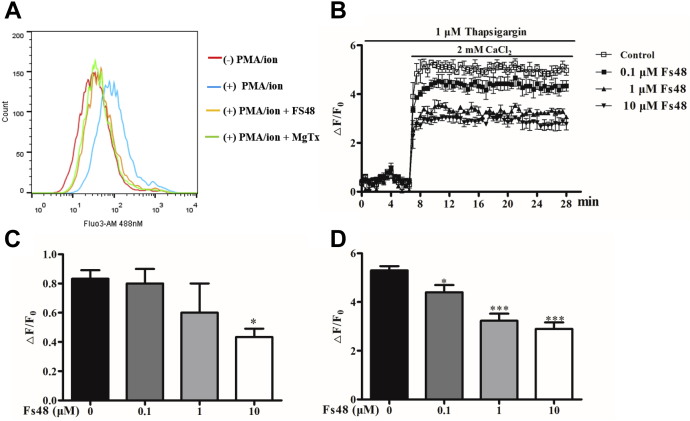
It has been proved that Kv1.3 channels play a critical role in the Ca2+ homeostasis of T cells by keeping the resting membrane potential, and the inhibition of Kv1.3 channel markedly decreases the Ca2+ influx (2, 3). To test this hypothesis, we used Fluo4-AM, a fluorescent probe widely used in measuring the content of intracellular Ca2+ to identify the effect of FS48 on changes of intracellular Ca2+ concentrations. Compared with the negative control, PMA/ionomycin induced a significant release of Ca2+ from the endoplasmic reticulum and rise in the cytoplasm of Jurkat T cells. However, when cells were treated by 3 μM FS48 and 100 nM MgTx, the increasing concentration of Ca2+ was reduced, which was indicated by the left shift of fluorescence signal compared with PMA/ionomycin-treated group (Fig. 3A). To further explore whether FS48 inhibited Ca2+ change of the cells, we measure Ca2+ concentration in response to TG and CaCl2 stimulation. As shown in Figure 3, B–D and Fig. S3, in Ca2+-free Ringer’s solution, treatment with 1 μM TG rose slightly the intracellular Ca2+ concentration. Incubation with 0.1 and 1 μM FS48 as well as 1 nM MgTx did not change the Ca2+ release induced by TG (p > 0.05), while 10 μM FS48 and MgTx at the concentration of 10 nM and 100 nM significantly inhibited this Ca2+ influx response (Figs. 3C and S3B). However, it was obviously observed that the readdition of Ca2+ to Ca2+-depleted cells induced significant intracellular Ca2+ increase after cell treatment with TG followed by addition of 2 mM CaCl2, which was concentration-dependently blunted by FS48 and MgTx at three tested concentrations by 25.36% ± 6.54%, 39.29% ± 4.39%, 64.29% ± 8.45% and 24.28% ± 2.15%, 49.46% ± 2.23%, and 53.51% ± 1.68% of the maximum Ca2+ influx, respectively (Figs. 3D and S3C). Taken together, these results demonstrate that FS48 like MgTx is a strong suppressor of intracellular Ca2+ signaling of T cells via blockage of Kv1.3 channel.
Figure 3.
Effect of FS48 on the Ca2+influx to Ca2+-depleted Jurkat T cells.A, flow cytometry analysis of the concentration of intracellular Ca2+ in Jurkat T cells. B, the summarized time course of the suppressed Ca2+ influx to Ca2+-depleted Jurkat T cells by FS48. Jurkat T cells were incubated with Fluo4-AM, resuspended in Ca2+ -free Ringer solution, and treated with 0, 0.1, 1, and 10 μM FS48 for 20 min before the intracellular Ca2+ release, and Ca2+ influx was elicited by TG and CaCl2, respectively. The fluorescence signals were read using microplate spectrophotometer. C, the ΔF/F0 value of the peak intracellular Ca2+ release response stimulated by TG. D, the ΔF/F0 value of the maximum Ca2+ influx response after 2 mM CaCl2 application. All data are presented as mean ± SD (n ≥ 3). ∗p < 0.05, ∗∗∗p < 0.001, compared with negative control group.
FS48 downregulated the Ca2+-related transcription factors
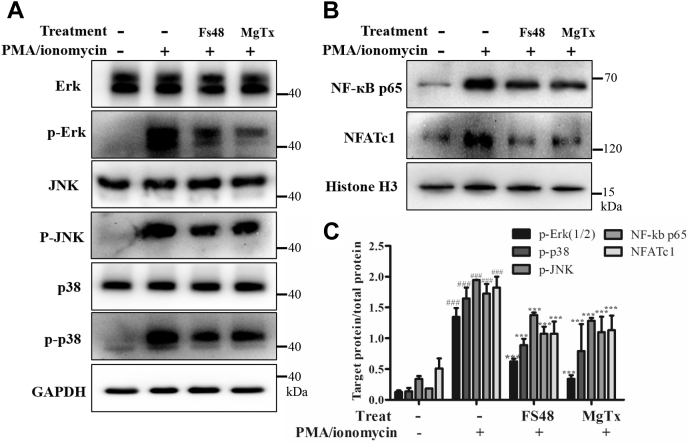
Ca2+ influx as well as Ca2+-related signal pathway factors, such as NF-кB, NFATc1 and MAPK (ERK, JNK and p38), mediate the activation of human T cells (2). To further explore the mechanism through which FS48 inhibited T cell activation, Western blot analysis was performed to investigate the effects of FS48 on the activation of NF-кB, NFATc1 and MAPK signaling pathways induced by PMA/ionomycin. Figure 4 showed that PMA/ionomycin treatment markedly increased nuclear translocation of NF-кB p65 and NFATc1 as well as the phosphorylation of ERK, JNK as well as p38. However, both 3 μM FS48 and 100 nM MgTx significantly moderated the phosphorylation of ERK, JNK, and p38 and the nuclear translocation of NF-кB p65 and NFATc1.
Figure 4.
Effect of FS48 on the activation of MAPK/NF-κB/NFATc1 pathway. Jurkat T cells were pretreated with 3 μM FS48 and 100 nM MgTx for 1 h, then the cells were stimulated with PMA/ionomycin (50 ng/ml; 1 μg/ml) for 4 h before collected for Western blot analysis. A and B, representative Western blot analysis of NF-κB p65 and NFATc1 nuclear translocation, ERK, JNK, and p38 phosphorylation. C, the ratios of p65 and NFATc1 protein to Histone H3 and phosphorylated ERK, JNK, and p38 to corresponding total protein. Quantity One software (Bio-Rad) was used for band density analysis. All data are shown as mean ± SD (n ≥ 3). ###p < 0.001, compared with negative control group; ∗∗∗p < 0.001 compared with model group.
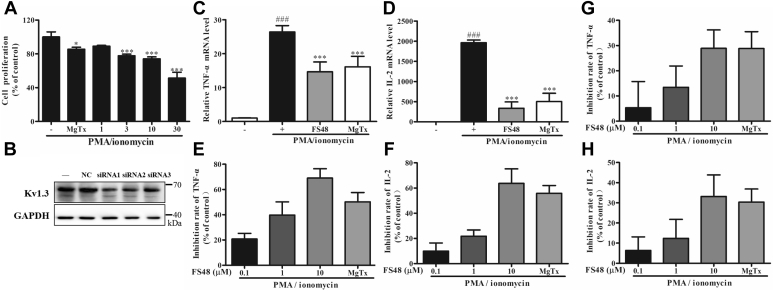
FS48 reduced cell proliferation and IL-2 as well as TNF-α production
In order to determine whether the inhibition of Kv1.3 channel and Ca2+ signaling pathways by FS48 might cause the immunoregulatory effect as was reported for Acacetin and Lovastatin (5, 6), we further investigated the effect of FS48 on the proliferation and expression of TNF-α and IL-2 from PMA/ionomycin-triggered Jurkat T cells. As illustrated in Figure 5, both FS48 and MgTx suppressed TNF-α and IL-2 mRNA expression stimulated by PMA/ionomycin. Moreover, both MgTx and FS48 also inhibited the cell proliferation and secretion of TNF-α and IL-2 protein in supernatant with a concentration-dependent manner. We found that treatment with 0.1, 1, 10 μM FS48, and 100 nM MgTx resulted in secretion inhibition of 20.74% ± 4.52%, 39.68% ± 10.50%, 69.04% ± 7.41%, 50.23% ± 7.32% TNF-α and 9.89% ± 6.49%, 21.75% ± 4.96%, 63.73% ± 11.49%, 55.78% ± 6.25% IL-2, respectively. Furthermore, in order to verify whether the inhibition of TNF-α and IL-2 secretion by FS48 was related to Kv1.3 channel, we suppressed Kv1.3 expressions with specific Kv1.3 siRNA in Jurkat T cells. We found that siRNA1 was the most effective siRNA and caused 56.67% ± 4.11% knockdown of Kv1.3 channel, while siRNA-NC caused no apparent change. Importantly, although FS48 and MgTx also decreased TNF-α and IL-2 secretion in Kv1.3 knockdown Jurkat T cells in concentration-dependent manner, their inhibitory effects were significantly reduced and the same concentration of FS48 and MgTx inhibited 5.31% ± 8.48%, 13.4% ± 6.92%, 28.9% ± 5.99%, 28.81% ± 5.47% TNF-α secretion and 6.34% ± 5.51%, 12.35% ± 7.69%, 33.15% ± 8.75%, 30.37% ± 5.30% IL-2 secretion after knockdown of Kv1.3 channel. These results indicated that FS48 exerts its immunosuppression effect most likely through Kv1.3 channels and may be a new lead drug template for autoimmune diseases.
Figure 5.
Effect of FS48 on the secretion of TNF-α and IL-2 in Jurkat T cells stimulated with PMA/ionomycin.A, effect of FS48 (1, 3, 10, 30 μM) and 100 nM MgTx on the proliferation of Jurkat T cells stimulated with PMA/ionomycin. B, knockdown of Kv1.3 channel expression with different siRNA. C and D, the mRNA production of TNF-α and IL-2. E and F, the inhibition rate of TNF-α and IL-2 secretion in Jurkat T cells. G and H, the inhibition rate of TNF-α and IL-2 in Jurkat T cells after knockdown Kv1.3. All data are presented as mean ± SD (n ≥ 3). ###p < 0.001, compared with negative control group; ∗p < 0.05, ∗∗∗p < 0.001 compared with model group.
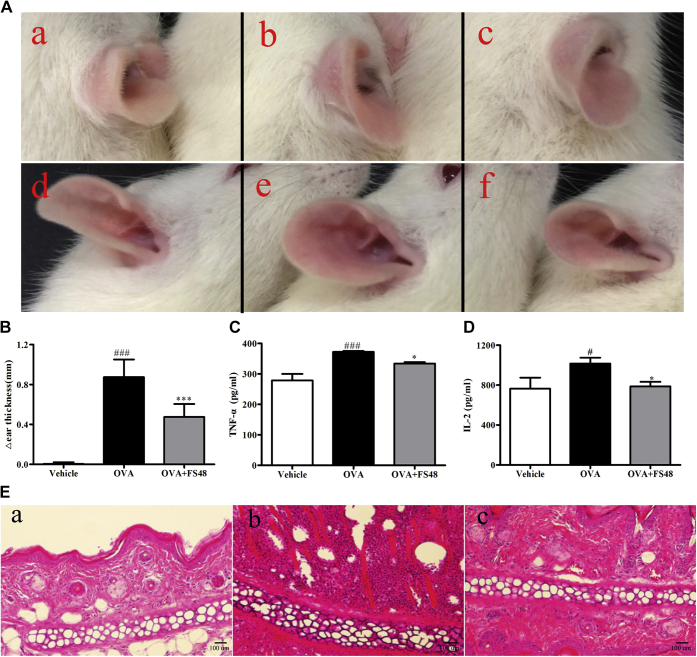
FS48 attenuated DTH reactions in vivo
The DTH reaction in which the cell-mediated pathologic response involves T cell activation is a perfect model investigating the in vivo effects of Kv1.3 blockers (1, 13). Thus, we evaluated the immunosuppressive activity of FS48 in a rat model of DTH. When compared with the control ears, OVA induced a pronounced swelling caused by inflammatory reaction in the challenged ear. However, when treated with FS48 and MgTx, ear swelling induced by OVA was markedly ameliorated by 47.33% ± 5.32% (Figs. 6, A and B and S1). Consistent with the measurement results of ear thickness, TNF-α and IL-2 expressions in OVA-changed tissues were also increased in the model group (Fig. 6, C and D). In addition, histological sections of auricle tissue showed that the OVA-challenged ears of DTH rats suffered from edema and infiltration of leukocytes into the epidermis and dermis, which were significantly suppressed by FS48 (Fig. 6E). Together, these results suggest that FS48 could significantly suppress the OVA-induced DTH reaction probably through inhibiting lymphocyte function.
Figure 6.
DTH immunosuppressive effects of FS48.A, photographic images of rat ear edemas. Photographs at 24 h postinjection show the gross pathology of ear from saline (a and d), OVA plus saline (b and e), OVA plus 2.5 mg/kg FS48 (c and f) groups. The top and lateral views of ears are displayed in parts a–c and d–f, respectively. B, the thickness increase between the OVA-challenged ear and the saline-treated ear in each group (n = 6) 24 h after the second challenge. C and D, the summarized secretion of TNF-α and IL-2 in the ear tissue. E, histology analysis of rat auricle tissue sections in vehicle (Panel a), OVA-treated (Panel b) and OVA +2.5 mg/kg FS48-treated DTH rats (Panel c) in 24 h (Original magnification, ×100). Data were showed as means ± SD, ###p < 0.001 compared with vehicle group; ∗p < 0.05 and ∗∗∗p < 0.001 compared with model group.
Discussion
Kv1.3 constitutes the main K+ channel expressed in human T cells and plays a crucial role to maintain the resting membrane potential, which provides electrochemical driving force for Ca2+ influx. Blocking Kv1.3 channel can cause the depolarization of the membrane potential (17), decrease of Ca2+ influx, inhibition of the gene expression of IL-2 and TNF-α, and finally suppression of the proliferation and activation of T cells (1, 2, 7, 9, 18, 19). These characteristic features are clearly demonstrated by Kv1.3 peptide inhibitors from scorpions (CHTX, KTX, and MgTx) (1, 9, 17, 20, 21), and from sea anemones (ShK) (22). Similar with these Kv1.3 blockers (4, 8, 9, 23), FS48 at tested concentrations displays inhibitory effects on the depolarization of the membrane potential, Ca2+ influx, the Ca2+-dependent activation of MAPK/NF-κB/NFATc1 signal, T cell proliferation, and production of IL-2 as well as TNF-α stimulated by PMA/ionomycin, which can be significantly attenuated by siRNA knockdown of Kv1.3 channels (1, 24). Furthermore, FS48 can also suppress Kv1.3 protein expression after 24 h incubation with activated Jurkat T cells (Fig. 2, C and D), which will extend the blocking effect of FS48 on Kv1.3 channels and damage the function of T-cell. Finally, FS48 ameliorates the DTH response in rats. To conclude, FS48 can decrease T-cell activation in T-cell-mediated pathological conditions by blocking the Kv1.3 currents and channel protein expression in vivo.
Zhi Li proposes that a calcium-dependent pathway might be involved in the transcription of KCNA3 gene (23). Thus, the upregulation of the Kv1.3 channel may be driven by Ca2+ influx and suppressed by channel inhibitors due to a cross talk between channel activity and channel expression in T cells. Consistently, our data (Fig. 2, B–D) have demonstrated a correlation between enhanced intracellular Ca2+ concentration and Kv1.3 expression in activated T cells (4, 23). Interestingly, it has been previously demonstrated that pretreatment with ADWX-1, Diclofenac, and 18β-GA for 24 h shows no significant effect on KCNA3 mRNA level in nonactivated T-cells but significantly reduces its increase in activated T cells with increased intracellular Ca2+ concentration (4, 23, 25). Consistent with the effects of these inhibitors in nonactivated T cells, our data in Fig. S4 demonstrate that neither FS48 nor MgTX regulates KCNA3 gene transcription in Jurkat T cells without PMA/ionomycin stimulation. Such an effect for MgTx has already been reported by Conforti (26). Our data also demonstrate that FS48 and MgTx can’t lower the enhanced KCNA3 mRNA expression in Jurkat T cells stimulated by PMA/ionomycin. More specifically, both FS48 and MgTx at tested concentrations can’t abolish the PMA/ionomycin-induced Ca2+ influx as judged from the flow cytometry analysis of the intracellular Ca2+ concentration in Jurkat T cells (Fig. 3A). As a result, residual Ca2+ increase consequently promotes upregulation of KCNA3 gene transcription, thus proving that FS48 and MgTx can’t decrease the increasing KCNA3 mRNA expression in Jurkat T cells stimulated by PMA/ionomycin. Remarkably, Villalonga et al. (25) have reported that PHA/PMA can’t change the mRNA and protein levels of Kv1.3 in Jurkat T cells, and it is known that Sp1 transcription factor regulates Kv1.3 expression by directly binding to the promoter region of KCNA3 gene in the nucleus of A549 cells (27). Therefore, other complex regulation mechanisms unrelated to Ca2+ concentration maybe be also responsible for the transcription regulation of KCNA3 gene. It has also been previously demonstrated that the expression regulation of Kv1.3 protein takes place at a posttranscriptional level, independent of changes in mRNA expression (5, 7, 26, 28). As a result, the decreased expression of Kv1.3 channels in activated T cells is not in agreement with its mRNA levels. In this study, our data suggest that downregulation of Kv1.3 channel expression by FS48 treatment occurs posttranscriptionally, similar to the effects reported for hypoxia, DPO-1 and Acacetin, which can also lower Kv1.3 expression in the protein level with unchangeable mRNA expression (5, 7, 26). It is known that (a) pretreatment of Jurkat T cells with DPO-1 for 24 h rather than 30 min significantly reduces the Kv1.3 channel protein expression but (b) 30 min incubation with DPO-1 also inhibits the Ca2+ influx in Ca2+-depleted Jurkat T cells (7), thus suggesting a different mechanism of action between DPO-1 and FS48. Phosphorylation mechanisms can affect protein synthesis at the initiation or elongation phase (29). To conclude, changes in channel protein synthesis, degradation, or other posttranscriptional modification are likely responsible for the observed decreased Kv1.3 expression in PMA/ionomycin-stimulated Jurkat T cells after FS48 treatment for 24 h. MgTx, like Lovastatin, does not change the expression of Kv1.3 channels in nonstimulated Jurkat T cells at both the mRNA and protein levels (Fig. S4) (6, 7, 26), but the specific peptide also shows similar lack of effects in the stimulated Jurkat T cells (Figs. 2, C and D and S2), a finding that makes the mechanism of MgTx action different from that induced by FS48 in the stimulated Jurkat T cells. Therefore, our data suggest a different posttranscriptional modification and regulation of the Kv1.3 expression in the presence of FS48 (when compared with MgTx), and further research is necessary to investigate the molecular mechanism, which mediates the observed difference in the action of MgTx and FS48.
It is reported that Kv1.3 channel expression is closely associated with the MAPK/NF-κB/NFATc1 signaling activation (30, 31, 32, 33, 34, 35). Here, the activation of MAPK/NF-κB/NFATc1 pathways in Jurkat T cells stimulated by PMA/ionomycin can be significantly suppressed by FS48 (Fig. 4), which is in line with its effects on macrophages and the effects of other Kv1.3 blockers on TEM cells (30, 31, 32, 33, 34, 35). Three activated kinases of MAPK pathway result in a cascade of downstream transcription factors that, in turn, cause T cell proliferation and the expression activation of Kv1.3. The suppression function of FS48 on signal cascade may partly explain the observed inhibition of Jurkat T cell proliferation and the Kv1.3 expression stimulated by PMA/ionomycin. The activation of MAPK pathway is necessary during DTH (36, 37), which directly or indirectly mediates the production of inflammatory cytokines such as TNF-α in vivo (38, 39, 40). The Kv1.3 blocking peptides such as MgTx, KTX, ShK-186, and BmKTX-D33H ameliorate autoimmune DTH reaction characterized by tissue swelling and infiltration in the subcutaneous layer and have no obvious side effects in vivo (9, 13, 22, 41, 42, 43). Consistent with these findings and its effects in Jurkat T cells, like MgTx, FS48 markedly suppresses the ear swelling (Figs. 6, A and B and S1), the production of TNF-α and IL-2 and inflammatory infiltrate in an OVA-induced DTH response (Fig. 6, C–E). These results further demonstrate that the immunoregulatory properties of FS48 on MAPK signal cascades. Furtherly, considering that FS48 displays anti-inflammatory effect in vivo via Kv1.3 channel blockage and expression suppression as well as MAPK/NF-κB pathway inactivation of macrophage, which is one important cellular component of DTH (16, 44, 45), we can speculate that the effects of FS48 on DTH should be due to its combined effects on two or more kinds of cells expressing Kv1.3 channels on their membranes. Notably, despite the fact that FS48 is a weak blocker of Kv1.3 channel when compared with the above toxin peptides which are known of displaying DTH suppression effects in vitro, it shows anti-inflammatory and immunomodulatory effects in vivo, which are relatively stronger in comparison with MgTx, KTX, ShK-186, and BmKTX-D33H (8, 9, 13, 22, 41, 42, 43). Two reasons may be responsible for this phenomenon. Firstly, given the lack effects of MgTx on the Kv1.3 expression, the channel expression inhibition mediated by FS48 in immune cells such as T-cells may strengthen its immunosuppression potential in vivo. Secondly, the higher immunogenicity or/and lower stability of MgTx when compared with FS48 might reduce the therapeutic efficacy in vivo. As shown by Fig. S5 and Tables S1–S3, which are derived from our bioinformatics-based prediction of B cell and T cell epitopes, MgTx has greater immunogenicity than FS48. Furthermore, the plasma half-life time of FS48 was about 353.1 min (Fig. S6), which is longer than the 120 min observed for MgTx by Koo et al. (42). Therefore, FS48 maybe be more stable than MgTx in vivo. However, further research with experimental animals treated by flea FS48 and MgTx is necessary to ensure the exact mechanism responsible for this phenomenon. Anyway, this still proves a therapeutic potential of FS48 as an ideal candidate molecule against human T-cell-mediated inflammatory diseases such as DTH, rheumatoid arthritis, and psoriasis, which can be reversed by Kv1.3 blockers in vivo (10, 46).
Taken together, our data suggest that FS48 exhibits an immunosuppressive effect in vitro and in vivo, probably by inhibiting proliferation and activation of lymphocytes via blocking Kv1.3 channels. This is the first report that a protein from the salivary gland of haematophagous arthropods can be involved in immunomodulation of T-cell-mediated immune response, which contributes to explain the blood-sucking mechanism of X. cheopis.
Experimental procedures
Materials and chemicals
RPMI-1640, fetal bovine serum, phosphate buffer saline (PBS), and penicillin/streptomycin were all from Gibco. Phorbol ester (PMA)/ionomycin, thapsigargin (TG), and fluo-4 AM were purchased from Sigma-Aldrich, Alomone Laboratories, and Invitrogen, respectively. All liquid stock was kept light protected in −20 °C. The human Jurkat T cell line was purchased from the China Center for Type Culture Collection, cultured with complete RPMI-1640 contained 10% FBS and 1% streptomycin/penicillin, and maintained at 37 °C under 5% CO2 atmosphere. MgTx and primary antibodies against Kv1.3 were from Alomone Labs, p38, p-p38, ERK, p-ERK, JNK, p-JNK, Histone H3, NF-кB p65, NFATc1, GAPDH, and horseradish peroxidase–conjugated secondary antibodies were from Cell Signaling Technologies.
Electric recordings and analysis
Jurkat T cells were applied for the patch-clamp electrophysiological recordings in the whole-cell mode at 22 °C as previously described by us (47). The external solution was as follows (in mM): 5.9 KCl, 2.2 CaCl2, 137 NaCl, 1.2 MgCl2, 10 HEPES, and 14 D-glucose (adjusted to pH 7.3 with KOH) and the pipette solution contained (in mM): 140 KF, 2 Na2ATP, 4 MgCl2, 1 EGTA, and 10 HEPES (adjusted to pH 7.3 with KOH). Kv1.3 currents were evoked by a 200-ms depolarization to +40 mV from a holding potential of −80 mV. The pCLAMP 10.3 software and Igor pro were used to obtain and analyze data, respectively. The Hill equation for IC50 calculation as follows: Y = 100/(1 + 10ˆ((LogIC50 − X) × H)) (where y is the normalized response, X is the log of concentration, and H is the slope factor or Hill slope). For I/V curve measurements, recordings were performed with the activation potential to be from −50 mV to +40 mV with 10 mV increment steps. The data points in current–voltage relationships were fitted to the Boltzmann equation: I/Imax = 1/[1 + exp (V − V1/2)/k], where k is the slope factor and V1/2 is the voltage for half-maximum activation.
Cell viability measurement
Cell viability and proliferation were measured with cell counting kit-8 (CCK-8) method. For cell survival assay, 5 × 104/well Jurkat T cells were seed into a 96-well plate and incubated with FS48 (0.1, 1, 10, 30 μM) for 24 h. For cell proliferation assay, 5 × 104/well Jurkat T cells were seed into a 96-well plate and incubated with FS48 (0.1, 1, 10, 30 μM) or 100 nM MgTx for 30 min before incubation with PMA/ionomycin (50 ng/ml;1 μg/ml) for 24 h, the cells treated with PBS were used as a negative control. CCK-8 solution (10 μl) was applied into the wells for 3 h, and the absorbance value was read at 450 nm wavelength with a Microplate Reader (Tecan Company). The negative control group was calculated as 100% survival rate.
Quantitative real-time PCR
Jurkat T cells were seed into a 6-well plate and pretreated with 3 μM FS48 or 100 nM MgTx, a Kv1.3 channel blocker, for 1 h before incubation with PMA/ionomycin (50 ng/ml;1 μg/ml) for 8 h. The cells treated with PBS were used as a negative control. The total RNA of each group was extracted with Trizol and was used for the synthesis of cDNA with the reverse transcriptase (TaKaRa Biotechnology). Real-time PCR was carried out with Hieff qPCR SYBR Green Master Mix (Yeasen) in Light Cycler 480 system. In all experiments, a total of 40 amplification cycles was performed as previously reported (16). The primers sequences were listed in Table 1. The relative expression was quantified with 2−ΔΔCT method and normalized to the fold change of GAPDH mRNA. Each treatment was repeated at least three times.
Table 1.
Primers used for qRT-PCR
| Name | Forward | Reverse |
|---|---|---|
| TNF-α | 5′-CACAGTGAAGTGCTGGCAAC-3′ | 5′-AGGAAGGCCTAAGGTCCACT-3′ |
| IL-2 | 5′-AGAACTCAAACCTCTGGAGGAAG-3′ | 5′-GCTGTCTCATCAGCATATTCACA-3′ |
| KCNA3 | 5′-TGCGGTTCTTCGCTTGTC-3′ | 5′-GTCCATTGCCCTGTCGTT-3′ |
| GAPDH | 5′-GTGAAGGTCGGTGTGAACGGATT-3′ | 5′-GGAGATGATGACCCTTTTGGCTC-3′ |
Inflammatory cytokine measurement
Jurkat T cells were treated with FS48 (0.1, 1, and 10 μM) and 100 nM MgTx for 30 min before stimulated with PMA/ionomycin (50 ng/ml; 1 μg/ml) for 24 h. The cells treated with PBS were used as a negative control. Then, the supernatants were collected after centrifugation, and the concentration of TNF-α and IL-2 was determined with ELISA kit (Thermo Fisher Scientific Inc) according to the manufacturer’s instructions. Each experiment was repeated at least three times.
Western blot analysis
Jurkat T cells were cultured in 6-well plates and incubated with 3 μM FS48 and 100 nM MgTx for indicated time in the presence or absence of PMA/ionomycin, the cells treated with PBS were used as a negative control. The cells were lysed with RIPA buffer and prepared for Western blot analysis as previously reported (48). Primary antibodies against Kv1.3 (4 °C, 16 h, 1: 200), p65, p38, p-p38, ERK, p-ERK, JNK, p-JNK, Histone H3, NF-кB p65, NFATc1, GAPDH, (4 °C, 16 h, 1:1000), and horseradish peroxidase–conjugated secondary antibodies (26 °C, 1 h, 1:2000) were applied, respectively. Chemiluminescence signals were detected and captured with Kodak XAR film. GAPDH and Histone H3 were used as the internal reference for proteins in cytoplasm and cell nucleus, respectively. All experiments were carried out independently at least three times.
Intracellular calcium measurement
Intracellular Ca2+ concentration was measured with both flow cytometry and microplate reader assays. For flow cytometry assay, Jurkat T cells were cultured in a 6-well plate at a density of 1 × 106 cells/ml and respectively treated with 3 μM FS48 and 100 nM MgTx for 1 h before incubation with PMA/ionomycin (50 ng/ml; 1 μg/ml) for 6 h, the cells treated with PBS were used as a negative control. The cells were washed with PBS for three times and loaded with 5 μM Fluo4-AM for 45 min. Then, the cells were collected after centrifugation and resuspended with PBS. Intracellular Ca2+ concentrations were determined using the FACScan flow cytometer (Becton Dickinson Company) with excitation wavelength 488 nm and emission wavelength 525 nm, and a total of 10,000 events were collected of each sample. FlowJo (ver. 7.6) software was used to analyze the FACScan flow cytometry data. For microplate reader assay, Jurkat T cells were collected and washed with Ca2+ free Ringer’s solution, which were composed of (in mM): 155 NaCl, 5 HEPES, 4.5 KCl, 3 MgCl2, 10 Glucose, 1 EGTA. The cells were incubated with 2 μM Fluo4-AM in Ca2+ free Ringer’s solution for 30 min after washed twice, a total of 5 × 104/well cells were seed into a 96-well black plate and treated with FS48 (0.1, 1, 10 μM) for 20 min, the cells treated with PBS were used as a negative control. After incubation, the cells were added with 1 μM TG to elicit Ca2+ release in Ca2+ free Ringer’s solution, and fluorescent signals were read using microplate spectrophotometer (Tecan Company) with excitation wavelength 488 nm and emission wavelength 525 nm at 10 s intervals. At the fifth minute point, a final concentration of 2 mM CaCl2 was add to stimulate Ca2+ influx into the cell. The Ca2+ influx response was presents as ΔF/F0 (ΔF = F − F0, where F0 is the mean value of the background fluorescence value).
Knockdown of Kv1.3 expression with siRNA
Jurkat T cells were transfected with siRNA to knockdown the expression of Kv1.3 channel as described in our previous study (16). Western blot analysis was carried out to determine the efficiency of siRNA knockdown rate. The most effective siRNA was selected for further experiment. The cells were transfected with most effective siRNA for 24 h and further treated with FS48, MgTx, and PMA/ionomycin as described above, and the supernatants were used to measure the protein levels of TNF-α and IL-2 with ELISA kit according to the manufacturer’s instructions.
Prevention of active DTH
Eight-week-old female Wistar rats (180–200 g) were purchased from the Laboratory Animal Center of Southern Medical University and housed in plastic cages (relative humidity: 40%–70%; temperature: 20–26 °C) with a 12 h light/dark cycle and supplied with food and water ad libitum. The Animal Care and Use Ethics of Southern Medical University approved all protocols and procedures involving live animals in the present study. The rats were randomly divided into control, model, and FS48-treated groups. Model and FS48-treated groups were subcutaneously inoculated with 200 μl ovalbumin (1 mg/ml, OVA and complete Freund's adjuvant 1:1 mixed emulsification, Difco) in scruff of the neck to develop memory T cells in vivo. After 7 days, rats in model group and FS48-treated group were subcutaneously injected with saline (0.9% NaCl, 0.5 ml/rat) and 2.5 mg/kg FS48 in scruff of the neck respectively for 1 h and then were challenged again with OVA (20 μl, 1 mg/ml) in auricle. Another ear of each rat was injected with equal volume of saline as a control. The thickness of ear was recorded at 0 and 24 h after the second OVA injection using a spring-loaded micrometer (Mitutoyo), Δear thickness represents the inflammatory reaction and DTH severity. Finally, the ears were sheared after euthanasia and prepared for cytokines measurement with ELISA and HE staining analysis according to the manufacturer’s instructions.
Statistical analysis
Data analysis and plotting were performed with IBM SPSS Statistics 20.0 software (SPSS Inc). All results are presented as mean ± SD. The significance of three or more groups was analyzed with one-way ANOVA followed by Bonferroni’s test. The unpaired Student’s t test was used to determine significance between two experimental groups. Statistical significance was set at p < 0.05.
Data availability
All data presented and discussed are contained within the article.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
Author contributions
Q. Z., B. Z., and X. X. conceptualization; Q. Z. data curation; Q. Z., Z. D., and X. X. formal analysis; X. X. funding acquisition; Q. Z., W. L., Z. D., and J. C. investigation; Q. Z., W. L., and J. C. methodology; X. C. and X. X. project administration; X. C. resources; Q. Z., W. L., Z. D., B. Z., and J. W. software; X. C. and X. X. supervision; W. L. validation; J. W., X. C., and X. X. writing—original draft; X. C. and X. X. writing—review and editing.
Funding and additional information
This study was funded in part by the Chinese National Natural Science Foundation (No. 31772476, 31861143050, 31911530077 to X. X. and 82070038 to X. C.).
Edited by Mike Shipston
Supporting information
References
- 1.Cahalan M.D., Chandy K.G. The functional network of ion channels in T lymphocytes. Immunol. Rev. 2009;231:59–87. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feske S. Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 3.Desai R., Peretz A., Idelson H., Lazarovici P., Attali B. Ca2+-activated K+ channels in human leukemic Jurkat T cells. Molecular cloning, biochemical and functional characterization. J. Biol. Chem. 2000;275:39954–39963. doi: 10.1074/jbc.M001562200. [DOI] [PubMed] [Google Scholar]
- 4.Fu X.X., Du L.L., Zhao N., Dong Q., Liao Y.H., Du Y.M. 18beta-Glycyrrhetinic acid potently inhibits Kv1.3 potassium channels and T cell activation in human Jurkat T cells. J. Ethnopharmacol. 2013;148:647–654. doi: 10.1016/j.jep.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Zhao N., Dong Q., Fu X.X., Du L.L., Cheng X., Du Y.M., Liao Y.H. Acacetin blocks kv1.3 channels and inhibits human T cell activation. Cell. Physiol. Biochem. 2014;34:1359–1372. doi: 10.1159/000366343. [DOI] [PubMed] [Google Scholar]
- 6.Zhao N., Dong Q., Qian C., Li S., Wu Q., Ding D., Li J., Wang B., Guo K., Xie J., Cheng X., Liao Y., Du Y.M. Lovastatin blocks Kv1.3 channel in human T cells: A new mechanism to explain its immunomodulatory properties. Sci. Rep. 2015;5:17381. doi: 10.1038/srep17381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao N., Dong Q., Du L.L., Fu X.X., Du Y.M., Liao Y.H. Potent suppression of Kv1.3 potassium channel and IL-2 secretion by diphenyl phosphine oxide-1 in human T cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandy K.G., Norton R.S. Peptide blockers of Kv1.3 channels in T cells as therapeutics for autoimmune disease. Curr. Opin. Chem. Biol. 2017;38:97–107. doi: 10.1016/j.cbpa.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira I.S., Ferreira I.G., Alexandre-Silva G.M., Cerni F.A., Cremonez C.M., Arantes E.C., Zottich U., Pucca M.B. Scorpion toxins targeting Kv1.3 channels: Insights into immunosuppression. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019;25 doi: 10.1590/1678-9199-JVATITD-1481-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beeton C., Wulff H., Standifer N.E., Azam P., Mullen K.M., Pennington M.W., Kolski-Andreaco A., Wei E., Grino A., Counts D.R., Wang P.H., LeeHealey C.J., Andrew B.S., Sankaranarayanan A., Homerick D., et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17414–17419. doi: 10.1073/pnas.0605136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beeton C., Wulff H., Barbaria J., Clot-Faybesse O., Pennington M., Bernard D., Cahalan M.D., Chandy K.G., Beraud E. Selective blockade of T lymphocyte K(+) channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13942–13947. doi: 10.1073/pnas.241497298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koshy S., Huq R., Tanner M.R., Atik M.A., Porter P.C., Khan F.S., Pennington M.W., Hanania N.A., Corry D.B., Beeton C. Blocking Kv1.3 channels inhibits Th2 lymphocyte function and treats a rat model of asthma. J. Biol. Chem. 2014;289:12623–12632. doi: 10.1074/jbc.M113.517037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matheu M.P., Beeton C., Garcia A., Chi V., Rangaraju S., Safrina O., Monaghan K., Uemura M.I., Li D., Pal S., de la Maza L.M., Monuki E., Flugel A., Pennington M.W., Parker I., et al. Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block. Immunity. 2008;29:602–614. doi: 10.1016/j.immuni.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pennington M.W., Beeton C., Galea C.A., Smith B.J., Chi V., Monaghan K.P., Garcia A., Rangaraju S., Giuffrida A., Plank D., Crossley G., Nugent D., Khaytin I., Lefievre Y., Peshenko I., et al. Engineering a stable and selective peptide blocker of the Kv1.3 channel in T lymphocytes. Mol. Pharmacol. 2009;75:762–773. doi: 10.1124/mol.108.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pennington M.W., Czerwinski A., Norton R.S. Peptide therapeutics from venom: Current status and potential. Bioorg. Med. Chem. 2018;26:2738–2758. doi: 10.1016/j.bmc.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 16.Deng Z., Zeng Q., Tang J., Zhang B., Chai J., Andersen J.F., Chen X., Xu X. Anti-inflammatory effects of FS48, the first potassium channel inhibitor from the salivary glands of the flea Xenopsylla cheopis. J. Biol. Chem. 2021;296:100670. doi: 10.1016/j.jbc.2021.100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonard R.J., Garcia M.L., Slaughter R.S., Reuben J.P. Selective blockers of voltage-gated K+ channels depolarize human T lymphocytes: Mechanism of the antiproliferative effect of charybdotoxin. Proc. Natl. Acad. Sci. U. S. A. 1992;89:10094–10098. doi: 10.1073/pnas.89.21.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vennekamp J., Wulff H., Beeton C., Calabresi P.A., Grissmer S., Hansel W., Chandy K.G. Kv1.3-blocking 5-phenylalkoxypsoralens: A new class of immunomodulators. Mol. Pharmacol. 2004;65:1364–1374. doi: 10.1124/mol.65.6.1364. [DOI] [PubMed] [Google Scholar]
- 19.Hanson D.C., Nguyen A., Mather R.J., Rauer H., Koch K., Burgess L.E., Rizzi J.P., Donovan C.B., Bruns M.J., Canniff P.C., Cunningham A.C., Verdries K.A., Mena E., Kath J.C., Gutman G.A., et al. UK-78,282, a novel piperidine compound that potently blocks the Kv1.3 voltage-gated potassium channel and inhibits human T cell activation. Br. J. Pharmacol. 1999;126:1707–1716. doi: 10.1038/sj.bjp.0702480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin C.S., Boltz R.C., Blake J.T., Nguyen M., Talento A., Fischer P.A., Springer M.S., Sigal N.H., Slaughter R.S., Garcia M.L., Et A. Voltage-gated potassium channels regulate calcium-dependent pathways involved in human T lymphocyte activation. J. Exp. Med. 1993;177:637–645. doi: 10.1084/jem.177.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu L., Pennington M., Jiang Q., Whartenby K.A., Calabresi P.A. Characterization of the functional properties of the voltage-gated potassium channel Kv1.3 in human CD4+ T lymphocytes. J. Immunol. 2007;179:4563–4570. doi: 10.4049/jimmunol.179.7.4563. [DOI] [PubMed] [Google Scholar]
- 22.Chi V., Pennington M.W., Norton R.S., Tarcha E.J., Londono L.M., Sims-Fahey B., Upadhyay S.K., Lakey J.T., Iadonato S., Wulff H., Beeton C., Chandy K.G. Development of a sea anemone toxin as an immunomodulator for therapy of autoimmune diseases. Toxicon. 2012;59:529–546. doi: 10.1016/j.toxicon.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z., Liu W.H., Han S., Peng B.W., Yin J., Wu Y.L., He X.H., Li W.X. Selective inhibition of CCR7(-) effector memory T cell activation by a novel peptide targeting Kv1.3 channel in a rat experimental autoimmune encephalomyelitis model. J. Biol. Chem. 2012;287:29479–29494. doi: 10.1074/jbc.M112.379594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He X.H., Zeng Y.Z., Xu L.H., Sun H., Zhen L., Fang D.J. Influences of protein kinase C inhibitors on the expression of IL-2 and IFN-γ by human T-lymphocytes. Chin. J. Pathophysiol. 2002;5:522–525. [Google Scholar]
- 25.Villalonga N., David M., Bielanska J., Gonzalez T., Parra D., Soler C., Comes N., Valenzuela C., Felipe A. Immunomodulatory effects of diclofenac in leukocytes through the targeting of Kv1.3 voltage-dependent potassium channels. Biochem. Pharmacol. 2010;80:858–866. doi: 10.1016/j.bcp.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Conforti L., Petrovic M., Mohammad D., Lee S., Ma Q., Barone S., Filipovich A.H. Hypoxia regulates expression and activity of Kv1.3 channels in T lymphocytes: A possible role in T cell proliferation. J. Immunol. 2003;170:695–702. doi: 10.4049/jimmunol.170.2.695. [DOI] [PubMed] [Google Scholar]
- 27.Jang S.H., Byun J.K., Jeon W.I., Choi S.Y., Park J., Lee B.H., Yang J.E., Park J.B., O'Grady S.M., Kim D.Y., Ryu P.D., Joo S.W., Lee S.Y. Nuclear localization and functional characteristics of voltage-gated potassium channel Kv1.3. J. Biol. Chem. 2015;290:12547–12557. doi: 10.1074/jbc.M114.561324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Attali B., Romey G., Honore E., Schmid-Alliana A., Mattei M.G., Lesage F., Ricard P., Barhanin J., Lazdunski M. Cloning, functional expression, and regulation of two K+ channels in human T lymphocytes. J. Biol. Chem. 1992;267:8650–8657. [PubMed] [Google Scholar]
- 29.Proud C.G. Translation. Turned on by insulin. Nature. 1994;371:747–748. doi: 10.1038/371747a0. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Q.L., Wang T.Y., Li M., Shang Y.X. Alleviating airway inflammation by inhibiting ERK-NF-kappaB signaling pathway by blocking Kv1.3 channels. Int. Immunopharmacol. 2018;63:110–118. doi: 10.1016/j.intimp.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Kan X.H., Gao H.Q., Ma Z.Y., Liu L., Ling M.Y., Wang Y.Y. Kv1.3 potassium channel mediates macrophage migration in atherosclerosis by regulating ERK activity. Arch. Biochem. Biophys. 2016;591:150–156. doi: 10.1016/j.abb.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Marmol R., Comes N., Styrczewska K., Perez-Verdaguer M., Vicente R., Pujadas L., Soriano E., Sorkin A., Felipe A. Unconventional EGF-induced ERK1/2-mediated Kv1.3 endocytosis. Cell. Mol. Life Sci. 2016;73:1515–1528. doi: 10.1007/s00018-015-2082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehring G.R., Kerschbaum H.H., Eder C., Neben A.L., Fanger C.M., Khoury R.M., Negulescu P.A., Cahalan M.D. A nongenomic mechanism for progesterone-mediated immunosuppression: Inhibition of K+ channels, Ca2+ signaling, and gene expression in T lymphocytes. J. Exp. Med. 1998;188:1593–1602. doi: 10.1084/jem.188.9.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li T., Wong V.K., Yi X.Q., Wong Y.F., Zhou H., Liu L. Pseudolaric acid B suppresses T lymphocyte activation through inhibition of NF-kappaB signaling pathway and p38 phosphorylation. J. Cell. Biochem. 2009;108:87–95. doi: 10.1002/jcb.22230. [DOI] [PubMed] [Google Scholar]
- 35.Wei N., Li T., Chen H., Mei X., Cao B., Zhang Y.Y. The immunosuppressive activity of pseudolaric acid B on T lymphocytes in vitro. Phytother. Res. 2013;27:980–985. doi: 10.1002/ptr.4824. [DOI] [PubMed] [Google Scholar]
- 36.Engstrom L., Pinzon-Ortiz M.C., Li Y., Chen S.C., Kinsley D., Nelissen R., Fine J.S., Mihara K., Manfra D. Characterization of a murine keyhole limpet hemocyanin (KLH)-delayed-type hypersensitivity (DTH) model: Role for p38 kinase. Int. Immunopharmacol. 2009;9:1218–1227. doi: 10.1016/j.intimp.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Li T., Wang W., Zhao J.H., Zhou X., Li Y.M., Chen H. Pseudolaric acid B inhibits T-cell mediated immune response in vivo via p38MAPK signal cascades and PPARγ activation. Life Sci. 2015;121:88–96. doi: 10.1016/j.lfs.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 38.Campbell J., Ciesielski C.J., Hunt A.E., Horwood N.J., Beech J.T., Hayes L.A., Denys A., Feldmann M., Brennan F.M., Foxwell B.M. A novel mechanism for TNF-alpha regulation by p38 MAPK: Involvement of NF-kappa B with implications for therapy in rheumatoid arthritis. J. Immunol. 2004;173:6928–6937. doi: 10.4049/jimmunol.173.11.6928. [DOI] [PubMed] [Google Scholar]
- 39.Agrawal S., Gollapudi S., Su H., Gupta S. Leptin activates human B cells to secrete TNF-alpha, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J. Clin. Immunol. 2011;31:472–478. doi: 10.1007/s10875-010-9507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Q., Yu H., Zinna R., Martin K., Herbert B., Liu A., Rossa C.J., Kirkwood K.L. Silencing mitogen-activated protein kinase-activated protein kinase-2 arrests inflammatory bone loss. J. Pharmacol. Exp. Ther. 2011;336:633–642. doi: 10.1124/jpet.110.172395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beeton C., Barbaria J., Giraud P., Devaux J., Benoliel A.M., Gola M., Sabatier J.M., Bernard D., Crest M., Beraud E. Selective blocking of voltage-gated K+ channels improves experimental autoimmune encephalomyelitis and inhibits T cell activation. J. Immunol. 2001;166:936–944. doi: 10.4049/jimmunol.166.2.936. [DOI] [PubMed] [Google Scholar]
- 42.Koo G.C., Blake J.T., Talento A., Nguyen M., Lin S., Sirotina A., Shah K., Mulvany K., Hora D.J., Cunningham P., Wunderler D.L., McManus O.B., Slaughter R., Bugianesi R., Felix J., et al. Blockade of the voltage-gated potassium channel Kv1.3 inhibits immune responses in vivo. J. Immunol. 1997;158:5120–5128. [PubMed] [Google Scholar]
- 43.Ye F., Hu Y., Yu W., Xie Z., Hu J., Cao Z., Li W., Wu Y. The scorpion toxin analogue BmKTX-D33H as a potential Kv1.3 channel-selective immunomodulator for autoimmune diseases. Toxins (Basel) 2016;8:115. doi: 10.3390/toxins8040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z., Chen Z., Chen T., Yi T., Zheng Z., Fan H., Chen Z. Berberine attenuates inflammation associated with delayed-type hypersensitivity via suppressing Th1 response and inhibiting apoptosis. Inflammation. 2017;40:221–231. doi: 10.1007/s10753-016-0472-6. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi K., Kaneda K., Kasama T. Immunopathogenesis of delayed-type hypersensitivity. Microsc. Res. Tech. 2001;53:241–245. doi: 10.1002/jemt.1090. [DOI] [PubMed] [Google Scholar]
- 46.Serrano-Albarras A., Estadella I., Cirera-Rocosa S., Navarro-Perez M., Felipe A. Kv1.3: A multifunctional channel with many pathological implications. Expert Opin. Ther. Targets. 2018;22:101–105. doi: 10.1080/14728222.2017.1420170. [DOI] [PubMed] [Google Scholar]
- 47.Xu X., Zhang B., Yang S., An S., Ribeiro J.M., Andersen J.F. Structure and function of FS50, a salivary protein from the flea Xenopsylla cheopis that blocks the sodium channel NaV1.5. Sci. Rep. 2016;6:36574. doi: 10.1038/srep36574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng Z., Chai J., Zeng Q., Zhang B., Ye T., Chen X., Xu X. The anticancer properties and mechanism of action of tablysin-15, the RGD-containing disintegrin, in breast cancer cells. Int. J. Biol. Macromol. 2019;129:1155–1167. doi: 10.1016/j.ijbiomac.2019.01.073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented and discussed are contained within the article.