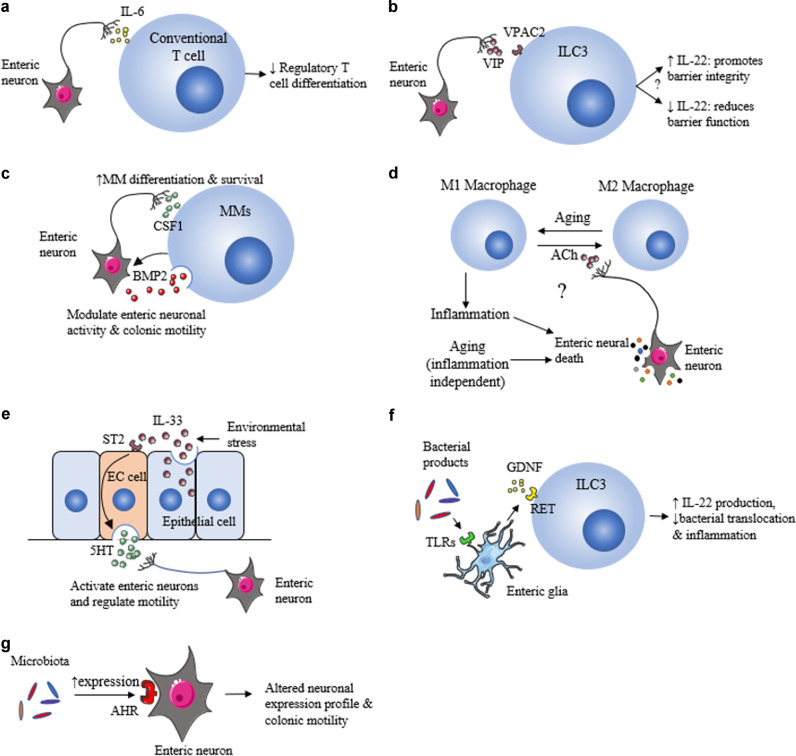
Fig. 2. Enteric neuroimmune interactions during homeostasis.
a IL-6 derived from enteric neurons reduces regulatory T cell differentiation. b VIP secreted by enteric neurons can bind to VIP receptor type 2 (VPAC2) expressed by ILC3s. VIP promotes or suppress IL-22 release from ILC3s, resulting in increased or decreased barrier function, respectively. c Enteric neurons secrete CSF1 to promote the survival and differentiation of muscularis macrophages (MM). In turn, MMs release bone morphogenic protein (BMP) 2 that excites enteric neurons and regulates colonic motility. d Hypothetical interactions between MM and enteric neurons during aging. Aging promotes phenotypical switches of MM from M2 to M1, leading to intestinal inflammation and consequent neuronal death. Aging itself can also cause neuronal loss, especially for cholinergic neurons. This may exacerbate inflammation as ACh can promote MM phenotypic switch to M2. e Environmental stress results in epithelial cells release of IL-33, which bind to ST2 receptors on enterochromaffin (EC) cells and promotes the release of 5-HT. Increased 5-HT modulates neuronal activities and increases intestinal motility. f Bacterial products bind TLRs on enteric glia leading to secretion of GDNF, which then binds to RET receptors on ILC3’s to promote IL-22 production. This limits bacterial translocation across the epithelium and reduces inflammation. g The microbiota promotes expression of AHR on enteric neurons, which in turn regulates the neuronal transcriptional profile and changes colonic motility. Creative Commons Attribution 3.0 Unported (CC BY 3.0).