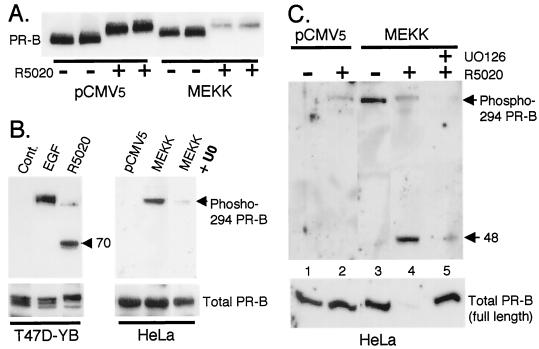
FIG. 4.
MEKK1 expression increases ligand-induced PR turnover and Ser294 phosphorylation. (A) MEKK1 augments PR protein turnover in the presence of progestins. Duplicate cultures of HeLa cells were transiently transfected with PR-B and either pCMV5 control vector or MEKK1 as described in Materials and Methods. Cells were treated without or with R5020 (10 nM) for 24 h, and PR protein levels in whole-cell lysates were determined with PR monoclonal antibodies. (B) Regulation of Ser294 phosphorylation in T47D-YB and HeLa cells. T47D-YB cells were treated with EGF (30 ng/ml) for 5 min or with R5020 (10 nM) for 1 h. HeLa cells were transfected with pCMV5 control vector or MEKK1 and treated without or with the MEK inhibitor (U0126) (20 μM) for 12 h. Phospho-Ser294 PR levels in cell lysates were measured with specific monoclonal antibodies to PR phospho-Ser294; a lower-molecular-weight phospho-Ser294-containing PR fragment of approximately 70 kDa in molecular mass was present in lysates from R5020-treated cells only (arrow). Total full-length PR levels remained constant (bottom); PR-B monoclonal antibodies failed to recognize PR fragments (not shown). (C) Regulation of Ser294 by MEKK1 plus R5020. HeLa cells were transfected with pCMV5 control vector or MEKK1 and pretreated without or with the MEK inhibitor, U0126 (20 μM), for 30 min prior to R5020 (10 μM) treatment for 12 h. Phosphorylated PR levels in cell lysates were measured with phospho-Ser294-specific monoclonal antibodies; a lower-molecular-weight phospho-Ser294-containing PR fragment of approximately 48 kDa was present in lysates from R5020-treated HeLa cells in the presence of MEKK1 (arrow). Total full-length PR levels were measured in the same lysates (lower panel); PR-B monoclonal antibodies failed to recognize PR fragments (not shown).