Summary
Background
In 2011, the World Health Organization began recommending glycated haemoglobin (HbA1c) as a measure for diagnosing type 2 diabetes (T2D). This initiative may have changed basic T2D epidemiology. Consequently, we examined time changes in T2D incidence and mortality during 1995-2018.
Methods
In this population-based cohort study, we included 415,553 individuals with incident T2D. We calculated annual age-standardized incidence rates of T2D. We examined HbA1c testing and used Poisson-regression to investigate all-cause mortality among the T2D patients and a matched comparison cohort from the general population over successive 3-year periods.
Findings
From 1995 to the 2012 introduction of HbA1c testing as a diagnostic option in Denmark, the annual standardized incidence rate (SIR) of T2D doubled, from 193 to 396 per 100,000 persons (4.1% increase annually). From 2012 onwards, the T2D incidence declined by 36%, reaching 253 per 100,000 persons in 2018 (5.7% decrease annually). This was driven by fewer patients starting treatment with an HbA1c measurement of <6·5% or without prior HbA1c testing. Mortality per 1,000 person-years following a T2D diagnosis decreased by 44% between 1995-1997 and 2010-2012, from 69 deaths to 38 deaths (adjusted mortality rate ratio: 0·55 (95% CI: 0·54-0·56)). After the low level during 2010-2012, mortality increased again by 27% to 48 per 1,000 person-years (95% CI: 46-50) by 2016-2018.
Interpretation
Our findings suggest that introducing HbA1c as a diagnostic option may have changed basic T2D epidemiology by leaving patients undiagnosed, that previously would have been diagnosed and treated.
Funding
Aarhus University funded the study and had no further involvement.
Research in Context.
Evidence before this study
There is now evidence from several high income countries in Europe and North America that the growth in diabetes prevalence has subsided and the incidence has begun to decrease. Recent landmark studies in the US and Scandinavia showed that all-cause mortality among adults with type 2 diabetes (T2D) has continuously declined until the late 2000s where it almost were approaching general population mortality. In 2011 the World Health Organization concluded that glycated haemoglobin (HbA1c) measurements could be used for type 2 diabetes diagnosis, co-existing with fasting blood glucose, and 2-hour oral glucose tolerance testing. There is limited overlap between which patients are diagnosed using the different testing options, and any impact of introducing HbA1c as de facto diagnostic method upon T2D population incidence and prognosis is poorly understood.
Added value of this study
From 1995 to the 2012 introduction of HbA1c testing as a diagnostic option in Denmark, the annual standardized incidence rate (SIR) of T2D doubled (4.1% annual increase). From 2012 onwards, the T2D incidence declined by 36% in 2018 (5.7% annual decrease). The decline was driven by a reduced number of patients who started glucose-lowering treatment with an HbA1c measurement below the new diagnostic threshold of <6·5% or without a previous HbA1c measurement. All-cause mortality following a T2D diagnosis decreased by 44% between 1995-1997 and 2010-2012. After the low level of mortality during 2010-2012, mortality increased again by 27% by 2016-2018. This was driven by an increase in mortality during the first year following T2D diagnosis.
Implication of all the available evidence
Our findings suggest the introduction of HbA1c as diagnostic option may have changed basic T2D epidemiology by leaving patients, that previously would have been treated, undiagnosed. We may thus be missing a group with borderline increased HbA1c values that is still at high metabolic risk and might benefit from a global cardiovascular risk assessment. These findings may have implications for clinical practice and suggest that a more multifactorial view of metabolic risk is needed.
Alt-text: Unlabelled box
INTRODUCTION
The estimated global type 2 diabetes (T2D) prevalence has increased from 108 million adults in 1980 to 422 million in 2014.1 It is predicted to nearly double by 2030-2045,2,3 but any projections are sensitive to developing trends in incidence of T2D and changes in mortality rates.
Diagnosis of T2D has traditionally relied on either fasting blood glucose (FBG) measurements or 2-hour oral glucose tolerance tests (OGTT).4,5 More convenient and robust diagnostic options have been pursued for decades, and in 2011 the World Health Organization concluded that glycated haemoglobin (HbA1c) measurements could be used for T2D diagnosis, as an alternative to the two established diagnostic methods. It furthermore concluded there was insufficient evidence to make any formal recommendation on the interpretation of HbA1c levels below 6.5%.4 However, in a screening study in primary care, HbA1c testing only identified 48% of those who were diagnosed with diabetes based on FBG or OGTT.6 Similarly, screening people with coronary heart disease for diabetes using both FBG, OGTT and HbA1c revealed that only 7% were diagnosed by all three methods.7 Nevertheless, HbA1c testing may have become the predominantly used diagnostic tool after a 2012 update of diagnostic recommendations by health authorities.8, 9, 10 There is now evidence from several high income countries in Europe and North America that the growth in diabetes prevalence has subsided and the incidence has begun to decrease.11 The impact of the recent introduction of HbA1c testing on both diabetes incidence and mortality is poorly understood. Studies from Denmark and other countries noted declining T2D incidence after the introduction of HbA1c testing as a diagnostic option, but generally lacked a combination of longitudinal HbA1c testing data and T2D incidence data to further explore the role of HbA1c testing.12, 13, 14, 15 Other recent studies included too few years after the introduction of HbA1c measurements for T2D diagnosis to reliably evaluate any impact on diabetes incidence.14, 15, 16, 17 Recent landmark studies in the US and Scandinavia showed that all-cause mortality among adults with T2D has continuously declined until the late 2000s where it almost approached general population mortality.18,19 However, the newest trend curves indicate that excess mortality from diabetes may have begun to rise again after the time of diagnostic HbA1c introduction.18, 19, 20
We aimed to investigate temporal changes in incidence and all-cause mortality among patients with incident T2D during 1995-2018. We compared these trends to mortality trends in the general population and examined the consequences of introducing HbA1c as a diagnostic option in 2012.
METHODS
Study design, setting, and participants
We conducted a population-based longitudinal study covering the entire population of Denmark (5·8 million inhabitants) based on national healthcare data for 1990-2018. All analyses involving HbA1c tests were limited to the population residing in Northern Denmark (1·8 million inhabitants), where these data were available. The Danish national healthcare system provides universal tax-supported healthcare, guaranteeing unfettered access to general practitioners and hospitals, and partial reimbursement for prescribed drugs. The unique personal civil registration number assigned to all Danish residents at birth or upon immigration allows for unambiguous linkage of data sources at the individual level.21
Data sources
We linked four existing population-based medical databases in our study.21 The Danish National Prescription Registry covers all prescriptions redeemed at any pharmacy in Denmark since 1994.21 The Danish National Registry of Patients (DNRP) contains data on dates of admission and discharge from all Danish non-psychiatric hospitals since 1977 and records of emergency and outpatient specialist clinic visits since 1995.21 Each hospital encounter is recorded in the DNRP with one primary diagnosis and potentially multiple secondary diagnoses and since 1994 coded using the International Classification of Diseases, Tenth Revision (ICD-10). The Clinical Laboratory Information System (LABKA) database contains laboratory results from tests ordered in primary care practices and hospitals in Northern Denmark from 1990 onwards.21 The Danish Civil Registration System (CRS)21 was established in 1968 and provides daily updates on the age, sex, vital status, and residency of all inhabitants. 21
Diabetes patients and general population comparators
Starting in 1995, we identified patients with incident T2D using either the date of their first-ever redemption of a glucose-lowering drug prescription (Anatomical Therapeutic Chemical classification system [ATC] code starting with A10) or the date of their first ever DNRP hospital contact with a diagnosis of diabetes or a diabetes complication (ICD-8 or ICD-10 codes starting with 249-250, 2515, E10-E15, O24, T383A, M142, G590, G632, H280, H334, H450, H360, N083), whichever occurred first. The applicable date was defined as the diabetes diagnosis date in our study. We excluded patients who had not resided in Denmark for at least one year prior to this date. To ensure inclusion of truly incident patients, we excluded those who were diagnosed with diabetes or redeemed any glucose-lowering drug before 1 January 1995. Patients who were diagnosed with diabetes or redeemed insulin before age 30 years (ATC code starting with A10A) or any glucose-lowering drug before age 15 years were excluded as likely having type 1 diabetes.22 Women who gave birth within nine months after a diabetes diagnosis were excluded as likely having gestational diabetes mellitus. Women who had pre-existing hospital diagnosed polycystic ovarian syndrome or who redeemed any metformin prescription (ATC code A10BA02) in combination with clomifen (ATC code G03GB02) within 12 months following a diabetes diagnosis were excluded as likely having polycystic ovarian syndrome. Some patients with other forms of diabetes, e.g. type 1 diabetes diagnosed in late adulthood at age 30+ years, latent autoimmune diabetes in adults (LADA), or rare monogenic diabetes types, were thus categorized as having type 2 diabetes in our study. On the T2D diagnosis date, we matched each patient with five comparators from the general Danish population based on age (year of birth) and sex, defining the patient's first treatment date as the index date of that patient's comparators. Comparators were subject to the same exclusion criteria as patients with diabetes.
Comorbidities and mortality
We used the DNRP to obtain information on any comorbid conditions included in the Charlson Comorbidity Index (CCI),21,23 registered within five years prior to or on the diagnosis/index date. We categorized the severity of comorbidity using the CCI score (excluding diabetes), adapted for use with hospital discharge data.23 We computed the total CCI score for each individual, defining four categories of comorbidity: a total score of 0 (no comorbidity), a total score of 1 (moderate comorbidity), a total score of 2 (severe comorbidity), or a total score of ≥ 3 (very severe comorbidity). The CRS was used to link data on all-cause mortality and migration status of each patient and comparator until the end of 2018.21
HbA1c
For each patient in Northern Denmark with available laboratory data, the latest available HbA1c measurement within one year before first T2D treatment was obtained from the LABKA database. We used the following values to categorize baseline HbA1c levels: no measurement available, <6·5%, 6·5-6·9%, 7·0-7·4%, 7·5-7·9%, 8·0-8·9%, 9·0-9·9%, and ≥10%.24
Statistical analysis
We first compiled descriptive characteristics for all T2D patients according to 3-year periods of diagnosis. To assess changes in incidence of T2D over time, we plotted standardized incidence rates (SIRs) of T2D for each year, standardized to the age and sex distribution of the population of Denmark in 2012. Next, we restricted the population to Northern Denmark where laboratory data were available and calculated and plotted incidence rates (IRs) of T2D associated with different baseline HbA1c categories. We modelled the incidence rates for all T2D patients by calendar year using a Poisson model. This was done separately for the periods separated by HbA1c’s introduction as a diagnostic option (1995-2011 and 2011-2018).
To evaluate temporal changes in all-cause mortality among incident T2D patients, for each calendar year we calculated and plotted the all-cause mortality risk during 365 day intervals: 0-1 years, 1-2 years, 2-3 years, 3-4 years, and 4-5 years after first T2D treatment, separately for men and women and age-standardized to the incident T2D population in 2012. Next, we followed T2D patients and their population comparators from the diagnosis/index date until death, migration, first T2D diagnosis (in comparators), or end of follow-up, whichever came first. We plotted the cumulative unadjusted mortality by year of diagnosis. We used a Poisson regression model to plot mortality rates per 1,000 person-years for T2D patients and comparators, using all available follow-up time (maximum 24 years). We then examined changes in all-cause mortality rates for 3-year periods, using the first period, 1995-1997, as the reference period and calculating mortality rate ratios (MRR) adjusted for changes over time in age, sex, and comorbidity (continuous CCI score). We plotted annual changes in diabetes incidence by age categories and mortality for diabetes patients and comparators. In a sensitivity analysis, we repeated the mortality rate ratio calculations replacing the Poisson model with a Cox regression model. In another sensitivity analysis we excluded all patients diagnosed using ICD-10 code E14 “other diabetes” (v n = 4,493), patients initiating insulin monotherapy between 30 and 40 years of age (n = 3,303), patients with pre-existing pancreatic disease (n = 3,148), and patients redeeming at least one glucocorticoid prescription 12 month prior to diagnosis (n = 35,135).
Role of the funding source
The study was funded by Aarhus University. The funder had no influence on any of the following: study design, analysis, interpretation, writing of the report or decision to submit the paper for publication.
RESULTS
Patient characteristics
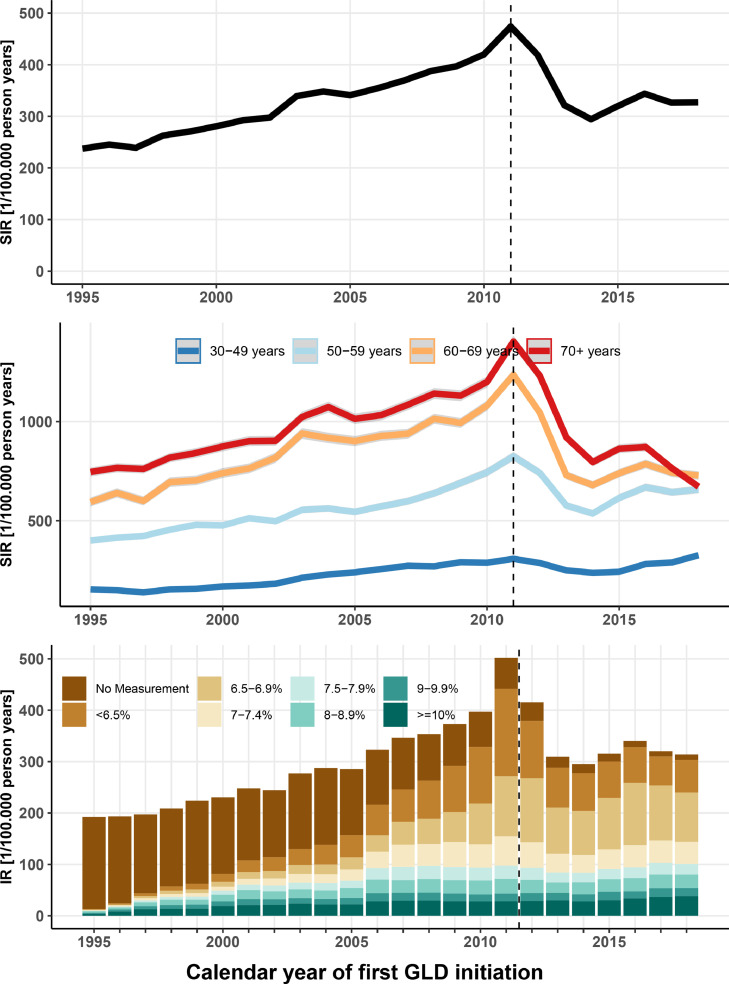
We identified 415,553 patients treated for T2D in Denmark for the first time from 1995 through 2018 and 2.060.279 matched comparators. For each 3-year period, baseline characteristics of the T2D patients at date of first treatment are presented in Table 1 and those of the comparators are presented in Table S1. Median age was 62·9 years (IQR: 52·9-72·5 years). We followed the T2D patients for a total of 2·7 million person-years. Median age at first T2D treatment fell from 63·7 years in 1995-1997 to 61·6 years in 2016-2018, while the sex distribution saw increasingly more men (55% to 57% male). The proportion with hospital-diagnosed comorbidity CCI score ≥2 increased from 14% to 28% during the study period. Median pre-treatment HbA1c values among T2D patients decreased substantially, from 8·7% in 1995-1997 to 7·0% in 2016-2018. A low median HbA1c level of 6·7% occurred in 2010-2012, when 25% of the patients (=lower quartile) had an HbA1c measurement of less than 6·3% (i.e., below the current diagnostic HbA1c threshold) at treatment initiation (Table 1).
Table 1.
Sex, age, comorbidity, and HbA1c values of type 2 diabetes patients in Denmark, by period of diagnosis.
| Period of diagnosis | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1995-1997 | 1998-2000 | 2001-2003 | 2004-2006 | 2007-2009 | 2010-2012 | 2013-2015 | 2016-2018 | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Overall | 34790 | 40068 | 46948 | 53897 | 61182 | 71653 | 51680 | 55328 |
| Sex | ||||||||
| Male | 19,036 (55) | 22,297 (56) | 26,132 (56) | 29,338 (54) | 33,713 (55) | 39,920 (56) | 29,120 (56) | 31,777 (57) |
| Female | 15,754 (45) | 17,771 (44) | 20,816 (44) | 24,559 (46) | 27,469 (45) | 31,733 (44) | 22,560 (44) | 23,551 (43) |
| Age (years) | ||||||||
| <50 | 6,730 (19) | 7,100 (18) | 8,379 (18) | 10,577 (20) | 12,089 (20) | 12,764 (18) | 10,130 (20) | 11,511 (21) |
| 50-59 | 7,600 (22) | 9,715 (24) | 11,526 (25) | 12,338 (23) | 13,208 (22) | 15,522 (22) | 11,656 (23) | 13,722 (25) |
| 60-69 | 8,265 (24) | 9,718 (24) | 11,995 (26) | 14,462 (27) | 17,687 (29) | 21,728 (30) | 14,075 (27) | 14,052 (25) |
| 70-79 | 8,024 (23) | 8,680 (22) | 9,489 (20) | 10,430 (19) | 11,874 (19) | 14,529 (20) | 10,428 (20) | 11,223 (20) |
| 80+ | 4,171 (12) | 4,855 (12) | 5,559 (12) | 6,090 (11) | 6,324 (10) | 7,110 (10) | 5,391 (10) | 4,820 (9) |
| Median (IQR) | 63.70 (52.50, 74.00) | 63.40 (53.30, 73.70) | 62.70 (53.60, 73.20) | 62.40 (52.90, 72.60) | 62.70 (52.80, 71.90) | 63.60 (53.70, 72.10) | 63.00 (52.50, 72.10) | 61.60 (51.80, 71.40) |
| Comorbidity | ||||||||
| No comorbidity | 24,951 (72) | 27,813 (69) | 31,951 (68) | 36,151 (67) | 39,762 (65) | 44,840 (63) | 30,576 (59) | 32,813 (59) |
| Moderate | 4,990 (14) | 6,040 (15) | 7,188 (15) | 8,179 (15) | 9,016 (15) | 10,296 (14) | 7,008 (14) | 7,037 (13) |
| Severe | 3,005 (9) | 3,674 (9) | 4,460 (9) | 5,138 (10) | 6,191 (10) | 7,487 (10) | 5,502 (11) | 5,847 (11) |
| Very severe | 1,844 (5) | 2,541 (6) | 3,349 (7) | 4,429 (8) | 6,213 (10) | 9,030 (13) | 8,594 (17) | 9,631 (17) |
| HbA1c (%)* | ||||||||
| No measurement | 9,032 (86) | 8,333 (70) | 7,524 (54) | 6,931 (43) | 4,899 (25) | 2,966 (13) | 992 (6) | 588 (3) |
| <6•5 | 165 (2) | 616 (5) | 1,448 (10) | 2,534 (16) | 4,088 (21) | 7,054 (30) | 3,992 (24) | 3,415 (19) |
| 6•5-6•9 | 113 (1) | 418 (3) | 821 (6) | 1,343 (8) | 2,727 (14) | 5,775 (24) | 4,970 (30) | 5,834 (33) |
| 7-7•4 | 138 (1) | 366 (3) | 750 (5) | 1,283 (8) | 2,424 (13) | 2,750 (12) | 1,956 (12) | 2,336 (13) |
| 7•5-7•9 | 119 (1) | 343 (3) | 628 (5) | 916 (6) | 1,390 (7) | 1,333 (6) | 1,033 (6) | 1,156 (7) |
| 8-8•9 | 254 (2) | 599 (5) | 911 (7) | 1,131 (7) | 1,392 (7) | 1,460 (6) | 1,232 (7) | 1,408 (8) |
| 9-9•9 | 225 (2) | 427 (4) | 590 (4) | 694 (4) | 806 (4) | 782 (3) | 787 (5) | 847 (5) |
| ≥10 | 447 (4) | 842 (7) | 1,181 (9) | 1,301 (8) | 1,581 (8) | 1,542 (7) | 1,610 (10) | 1,954 (11) |
| # measurements Median HbA1c (IQR) | 1,461; 8.70 (7.30, 10.40) | 3,611; 8.00 (6.80, 9.80) | 6,329; 7.60 (6.50, 9.20) | 9,202; 7.20 (6.40, 8.60) | 14,408; 7.00 (6.40, 8.00) | 20,696; 6.72 (6.30, 7.46) | 15,580; 6.91 (6.45, 7.82) | 16,950; 7.00 (6.54, 7.91) |
| First diagnosed using hospital diagnosis data n (%) | 14,679 (42) | 17,265 (43) | 20,497 (43) | 20,931 (39) | 19,096 (31) | 16,345 (23) | 13,059 (25) | 10,601 (19) |
| First diagnosed using prescription data n (%) | 20,118 (58) | 22,803 (57) | 26,441 (57) | 32,967 (61) | 42,095 (69) | 55,308 (77) | 38,617 (75) | 44,731 (81) |
Categories of comorbidity were based on Charlson Comorbidity Index (CCI) scores of 0 (no comorbidity), 1 (moderate), 2 (severe), and ≥3 (very severe); Diabetes was excluded from the CCI score. 30,233 (7.2%) of patients with incident diabetes had not redeemed a glucose lowering prescription before ultimo 2018.
HbA1c results are limited to persons who resided in Northern Denmark at the time of their T2D diagnosis.
Incidence
From 1995 to the 2012 introduction of HbA1c as a diagnostic option, the annual SIR of T2D per 100,000 people more than doubled from 193 to 396 (4.1% annualized increase). From 2011 to 2018, the annual SIR declined by 36% to 253 per 100,000 persons (Figure 1: top panel), with an overall low seen in 2014 (5.7% annualized decrease). The SIRs increased for men and women in all age groups until 2011, but the subsequent decline was predominantly observed in the older age groups (Figure 1: middle panel, and Figure S2: lower panel). Thus, in the age group ≥60 years, both men and women experienced a >50% decline in diabetes incidence from 2011 to 2014 (Figure 1: middle panel and Figure S2: middle panel). The decline in incidence was almost entirely driven by a reduction in the number of patients who started treatment with an HbA1c measurement below the new diagnostic HbA1c threshold of 6·5% or without a previous HbA1c measurement (Figure 1: bottom panel and Figure S4). After the 2014 low, the incidence increased more in men than in women (Figure S2: top panel).
Figure 1.
The top panel depicts age- and sex-standardized incidence rates (SIRs) among patients with incident type 2 diabetes with 95% confidence intervals by year of diagnosis. Similarly, the middle panel shows SIRs by age categories. The bottom panel shows the incidence rate stratified by baseline HbA1c measurement at time of first treatment among type 2 diabetes patients living in Northern Denmark at time of diagnosis. Incidence trends in this regional setting are similar to those on a national level.
Mortality
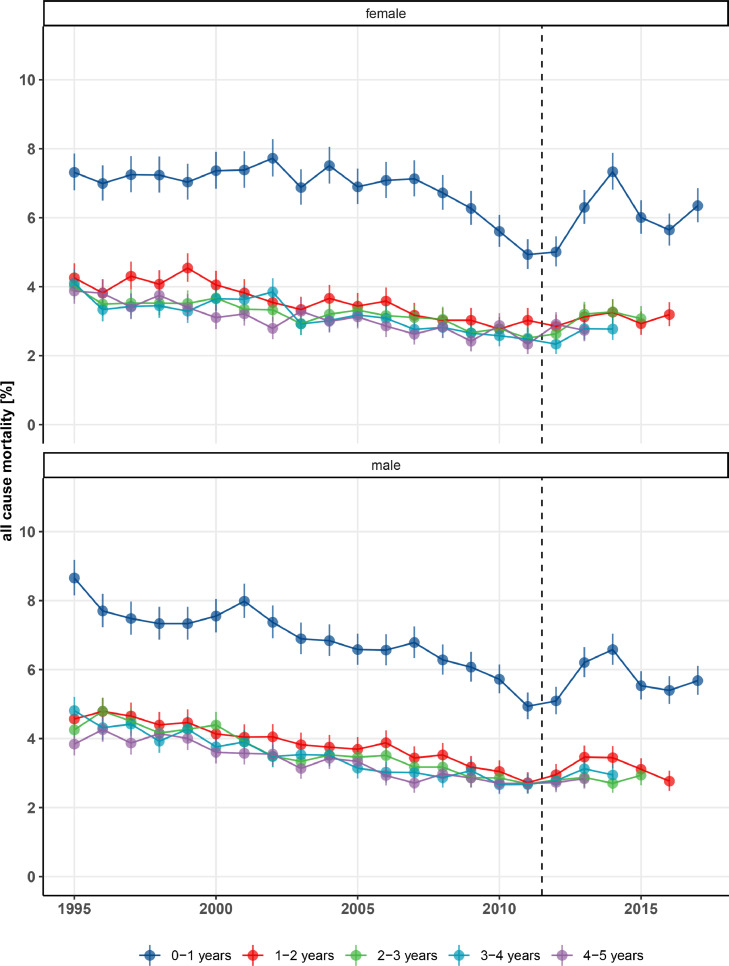
The all-cause mortality risks within 0-1 years, 1-2 years, 2-3 years, 3-4 years, and 4-5 years after first treatment were similar in men and women with T2D (Figure 2). The mortality risk within 0-1 year was clearly higher than subsequent one-year mortality risks. The 0-1 year mortality also showed the greatest variability over time, when findings before and after the diagnostic change in 2012 were compared (Figure 2). The adjusted mortality rate per 1,000 person-years among T2D patients decreased by 44%, from 72 deaths per 1,000 person-years during the reference period 1995-1997 to 40 deaths per 1,000 person-years during 2010-2012 (adjusted MRR: 0·55 [95% CI: 0·54-0·56]) (Table 2). After the low level of mortality in 2010-2012, mortality increased again by 27% to 48 per 1,000 person-years (95% CI: 46-50) during 2016-2018, corresponding to an adjusted MRR of 0·69 compared to the reference period 1995-1997 (Table 2). The reverted mortality trend after 2010-2012 was caused almost entirely by an increase in 0-1 year mortality (Figure 2). Figure S5 shows the annual mortality rates for T2D patients versus population comparators, adjusted by Poisson regression for age (60 years), sex (male), and comorbidities (CCI score 0). During 17 consecutive years before 2012, T2D mortality rates gradually converged between T2D patients and population comparators (Figure S5). In the following six years (until 2018), rates diverged again, caused by an increase in mortality in T2D patients and a continued decrease in mortality in the general population (Table 2, Figure S5). Figure S6 shows the successively decreasing cumulative mortality in T2D patients until 2010-2012 and increasing mortality hereafter. Figure S7 shows that the proportion of patients diagnosed with type 2 diabetes that had received an OGTT or FBG test quickly decreased after the 2011 introduction of HbA1c as a diagnostic test. Figure S8 shows that the year-to-year percentage changes in type 2 diabetes incidence and mortality were relatively stable until 2011, after which their variability increased much. Figure S12 shows that trends were generally similar across age groups.
Figure 2.
Age-standardized all-cause mortality by year in men and women with type 2 diabetes treated for the first time, Denmark, 1995-2018.
Table 2.
Mortality rate and mortality rate ratios over time for T2D patients and age- and sex-matched comparators.
| Type 2 diabetes patients |
Comparators | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period of diagnosis | Persons N | Risk time (years) | Events N | Mortality Rate/1000 py | rate ratio (95% CI) – crude | rate ratio (95% CI) – adjusted* | Persons N | Events N | Mortality Rate/1000 py | rate ratio (95% CI) - crude | rate ratio (95% CI) – adjusted* | |
| 1995-1997 | 34790 | 441244 | 24992 | 69.49 (67.31-71.73) | 1 (1-1) | 1 (1-1) | 172546 | 89778 | 35.69 (35.04-36.35) | 1 (1-1) | 1 (1-1) | |
| 1998-2000 | 40068 | 484218 | 25779 | 64.05 (62.06-66.12) | 0.94 (0.92-0.96) | 0.92 (0.91-0.94) | 199171 | 89779 | 33.96 (33.34-34.58) | 0.93 (0.93-0.94) | 0.95 (0.94-0.96) | |
| 2001-2003 | 46948 | 530348 | 25432 | 57.73 (55.93-59.59) | 0.85 (0.83-0.86) | 0.83 (0.82-0.85) | 233371 | 87018 | 32.55 (31.96-33.15) | 0.85 (0.85-0.86) | 0.91 (0.9-0.92) | |
| 2004-2006 | 53897 | 544638 | 23378 | 52.63 (50.97-54.34) | 0.76 (0.74-0.77) | 0.76 (0.74-0.77) | 267430 | 78797 | 30.26 (29.71-30.83) | 0.77 (0.76-0.77) | 0.85 (0.84-0.86) | |
| 2007-2009 | 61182 | 519800 | 19920 | 45.46 (44-46.96) | 0.68 (0.66-0.69) | 0.65 (0.64-0.67) | 303373 | 65827 | 27.19 (26.68-27.7) | 0.68 (0.68-0.69) | 0.76 (0.75-0.77) | |
| 2010-2012 | 71653 | 467392 | 16264 | 37.98 (36.73-39.27) | 0.61 (0.6-0.63) | 0.55 (0.54-0.56) | 355366 | 53872 | 24.15 (23.68-24.61) | 0.63 (0.62-0.64) | 0.68 (0.67-0.68) | |
| 2013-2015 | 51680 | 208681 | 8536 | 41.68 (40.17-43.25) | 0.72 (0.7-0.74) | 0.6 (0.58-0.62) | 255866 | 22602 | 21.1 (20.65-21.57) | 0.58 (0.57-0.59) | 0.59 (0.58-0.6) | |
| 2016-2018 | 55328 | 80428 | 3727 | 48.08 (46-50.26) | 0.82 (0.79-0.85) | 0.69 (0.67-0.72) | 273121 | 7419 | 19.33 (18.78-19.89) | 0.5 (0.49-0.52) | 0.54 (0.53-0.55) | |
Adjusted for age, sex and comorbidity. Abbreviations: py, person years; CI, confidence intervals.
DISCUSSION
We observed a doubling of the incidence of T2D in Denmark between 1995 and 2011, when HbA1c was first introduced as a primary diagnostic method. During the same period, mortality following first T2D dianggnosisgnosis was halved. Between 2012 and 2018, we found a marked decline in T2D incidence, driven by fewer elderly patients and fewer patients with a baseline HbA1c of <6·5%. In parallel, T2D mortality rates climbed back to pre-2012 levels. These results suggest that the shift to using HbA1c measurements as a diagnostic tool has had an important impact on diabetes incidence rates. In relation to estimating current diabetes mortality, it is important to note that the shift to HbA1c testing may have changed the character of the denominator, selecting a treated diabetic population at higher risk. Precise estimation of the direction of the T2D epidemic may require inclusion of laboratory data in the consideration of case definitions.
Comparison with other studies
Our population-based study used 24 consecutive years of data to examine associations between HbA1c measurements, T2D incidence, and all-cause mortality. The continuously increasing incidence and improving prognosis of T2D that we observed during the 2000s accords with findings from previous US,19,25 Norwegian,15 Swedish,17,18 Finnish,26 Danish,27, 28, 29 UK,30, 31 Portuguese32 and Australian33 studies.11 Most of these studies were based on T2D data from before the introduction of HbA1c measurements for diagnostic purposes25,26,33,34 or included only few data points following this change,14, 15, 16, 17, 18, 19,30,32 hampering assessment of subsequent changing in trends. A recent multi-country analysis suggested that the incidence of diagnosed diabetes is now stabilising or declining in many high-income countries.14 That study concluded that the reasons for the declines in the incidence of diagnosed diabetes warrant further investigation with appropriate data sources, which was a main objective of our study. We were unable to identify other similar population-based incidence studies that included long-term time trends of HbA1c levels at diagnosis. A recent Norwegian study reported declining T2D incidence during 2009-2014, similar to our findings, but did not include information on HbA1c levels or T2D mortality. In the US, where using HbA1c measurements for diagnosis was introduced as early as in 2010, a decline in T2D incidence began a few years earlier than observed in our study,12 indicating that reductions in T2D incidence might be partly caused by the introduction of HbA1c as a diagnostic option. A recent study from Denmark covering the period 1996-2016 similarly found that T2D incidence increased until 2011, declined until 2014, but seems to increase again after 2015.28 Our findings through 2018 corroborate and extend these results.
With few exceptions,30 previous studies have reported evolving mortality time trends among prevalent, not incident, T2D patients. A recent study from Denmark reported recently declining mortality among patients with prevalent T2D28 which includes mostly T2D patients diagnosed before the diagnostic changes in 2012. The population with prevalent T2D is important because it reflects the patient mix that most doctors see in everyday clinical care. The specific mortality rate in incident T2D patients is also important, and we are adding this evidence in our study. Several other studies comparing mortality trends in prevalent T2D patients versus general population comparators reported a convergence in mortality rates among T2D patients and comparison subjects in the years prior to the introduction of HbA1c as a diagnostic test,18,19,31 which corroborates our findings. A Swedish population-based study found that a continuous decline in all-cause mortality in prevalent diabetes patients began to reverse in 2010-2011, while mortality rates continued to decrease in matched controls.18 This is also in line with our findings. A UK study similarly reported all-cause mortality increases in T2D patients from 2012 to 2014, in contrast with a continued decline among controls.31 A US study based on the National Health Interview Survey reported a continuous T2D mortality decrease between 1988-1994 and 2010-2015, but pooling of the most recent years may have masked recent changes in mortality trends.19 Authors of previous studies that suggested increasing T2D mortality trends in most recent years generally abstained from commenting on the trends, possibly because too few data points were available to make an unambiguous assessment of the increases in mortality.
Strengths and limitations
We conducted a population-based cohort study in a setting with uniform access to health care, complete registration of hospital admissions, drug prescriptions, and laboratory data, and complete follow-up until death or emigration. This reduced selection biases stemming from selective inclusion of, e.g., specific hospitals, health insurance systems, or age groups.
Several limitations should be considered when interpreting our findings. Increased screening for T2D and earlier initiation of glucose-lowering drugs following T2D diagnosis35 would tend to temporarily inflate increases in T2D incidence, introducing a lead time bias resulting in apparent decreased mortality. There was high screening activity and a focus on early detection and intensive glucose control promoted by diabetes associations during the 2000s, which was then somewhat offset following the 2008 publication of the ADVANCE trial36 and a more conservative treatment approach. These mechanisms could offer an alternative hypothesis for the apparently transient increase and then fall in T2D incidence. In turn, during the 2010s new cardiology guidelines on HbA1c screening in patients with cardiovascular disease could have led to inclusion of a subgroup of more severely ill T2D patients than in the earlier years. This perhaps contributed to the trend of increased short-term mortality that we observed in the 2010s.
In addition, we could only identify and follow patients from the date of their first glucose-lowering drug treatment or first diabetes related hospital contact and had no means to assess T2D patients exclusively treated with changes in diet and lifestyle. Thus, in theory, trends could be affected by changes in clinicians’ decisions about whether or when to initiate pharmacological therapy. In the mid-2000s, the focus on early intensive T2D therapeutic intervention in primary care increased and the “watchful waiting” and solely diet/lifestyle change approaches declined. Accordingly, T2D individuals were increasingly more likely to be captured by community pharmacy prescription databases as the primary (first) data source from the mid-2000s, rather than being captured in hospital diagnosis databases first. The proportion first diagnosed using prescription data increased from 58% (1995-1997) to 81% (2016-2018). Since the vast majority of registered T2D individuals would be captured in both data sources after a shorter or longer delay, usually within the first years after diabetes registration,37 we do not believe these factors to cause major bias in incidence trends over time. We do not consider this a bias per se in our prognosis study, as earlier detection and initiation of therapy can be considered causal factors in the improvement of T2D prognosis over time. Still, recent data indicate that 15-18% of all diabetes patients known in primary care may not receive medical treatment and may not be captured by the included data sources,38 which is an inherent limitation of our study.
Some patients tested with HbA1c may still have been diagnosed with FBG. This study did not include complete information on FBG, HbA1c and OGTT, because tests from general practitioners analysed locally e.g. by use of point-of-care diagnostics are not included in the LABKA database. We did however examine trends in OGTT and FBG testing on the available data in order to address this limitation. We found sharp declines in the use of both tests following 2012 (Figure S7). We were able to adjust for changes in comorbidity during a period of 24 years, using diagnoses recorded in the DNRP (previously validated with positive predictive values exceeding 90%) and included in the CCI. Still, improved ascertainment of comorbidities over time may have contributed to more complete comorbidity adjustment in recent years and thus to an overestimation of mortality improvements compared with earlier years.
Generalizability, implications, and conclusions
The observed trends in T2D epidemiology in Denmark may apply to other high-income countries with similar trends in lifestyle risk factors and similar changes to T2D diagnosis and therapy guidelines in recent decades. It is made clear in recent guidelines that the HbA1c test is just one among several T2D diagnostic options aiming at identifying patients at increased risk of complications. These tests still include both OGTT testing and FBG testing. Nonetheless, HbA1c testing is clearly the most convenient method for patients and physicians in everyday clinical practice, as it requires no fasting and planning, has less day-to-day variability and causes less discomfort. Of note, all three options for T2D diagnosis and their thresholds have been validated by their ability to predict diabetic retinopathy, rather than mortality.4 There is currently much discussion about the fact that a considerable proportion of T2D patients may fulfil the diagnostic requirements of one method, but not the others.7 Patients diagnosed by different T2D diagnostic methods may represent different disease phenotypes or stages and thus have a different prognosis.39 In accordance with previous observations,6 our findings suggest that a significant proportion of incident T2D patients, with blood glucose in the diabetic range but normal (or pre-diabetic) HbA1c values of <6·5%, remained undiagnosed and untreated after 2012. In effect, this indicates that reported declines in T2D incidence may be an artefact resulting from a new diagnostic option. If that is the case, we might expect a later compensatory increase in T2D incidence when initially untreated T2D patients experience further increases in blood glucose and HbA1c values and are eventually diagnosed. Indeed, we observed a return to a trend of increasing T2D incidence, particularly in men, in the most recent years. However, more data are needed to evaluate whether this trend is transient.
The dramatic decline in registered T2D incidence starting in 2012 coincided with increasing early T2D mortality, possibly because increased use of HbA1c testing removed T2D patients with hyperglycemia but normal or pre-diabetic HbA1c values (and potentially better short-term prognosis) from the pool of treated T2D patients. One could argue that an increase in average T2D mortality with removal of anticipated “lower-risk” patients was expected when introducing HbA1c for T2D diagnosis. This might constitute an important healthcare problem only if these individuals have a materially elevated risk of cardiovascular events and death versus other individuals,40,41 and would have likely benefitted from T2D diagnosis and treatment.42 Of note, many individuals with prediabetes and diabetes have other metabolic disturbances and cardiovascular risk factors,18,42 regardless of the diagnostic definition for diabetes. There are good tools to quantify global cardiovascular risk, such as the recently revised SCORE equation.43 A recent regression discontinuity design study found that individuals with an incident HbA1c measure just above the T2D treatment threshold of 6·5% experienced a 21% lower rate of death or cardiovascular event than those with an HbA1c just below the threshold, indicating that patients with HbA1c just below the threshold are indeed a vulnerable patient group that might benefit from a global cardiovascular risk assessment and multifactorial treatment.44
In conclusion, we found that the incidence of T2D doubled while T2D mortality nearly halved between 1995 and 2011. After the 2012 shift in diagnostic policy, we saw a marked decline in T2D incidence and a higher mortality, probably driven by a shift in the case-mix of diagnosed T2D patients and fewer patients with a baseline HbA1c of <6·5% initiating T2D treatment. Our findings suggest that fewer patients have been diagnosed with T2D since HbA1c testing was introduced as a convenient diagnostic option. We may thus be missing a group with borderline increased HbA1c values that is still at high metabolic and cardiovascular risk.44 These findings may have implications for clinical practice and suggest that a more multifactorial view of metabolic risk is needed.
Acknowledgments
ACKNOWLEDGEMENTS
Author contributions: JSK, SSK, DRW, and RWT designed the study. JSK reviewed the literature. JSK, SSK, AH, DRW, and RWT directed the analyses, which were carried out by JSK, SSK and LP. All authors participated in the discussion and interpretation of the results. JSK organized the writing and wrote the initial draft. All authors critically revised the manuscript for intellectual content and approved the final version. RWT is the guarantor.
Role of funding source: Aarhus University funded the study. The funders had no role in the study design, data analysis, interpretation of data, or writing of the manuscript.
Conflicts of interests: All authors have completed the ICMJE Uniform Disclosure at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that they received no support from any organisation for the submitted work; no financial relationships in the previous three years with any organisations that might have an interest in the submitted work; and no other relationships or activities that could appear to have influenced the submitted work. AH and DW are supported by the Danish Diabetes Academy, which is funded by an unrestricted grant from the Novo Nordisk Foundation. Department of Clinical Epidemiology, Aarhus University Hospital, is a member of the Danish Centre for Strategic Research in Type 2 Diabetes (DD2), supported by the Danish Agency for Science (grant nos. 09-067009 and 09-075724), the Danish Health and Medicines Authority, the Danish Diabetes Association, and an unrestricted donation from Novo Nordisk A/S. Project partners are listed on the website www.DD2.nu. Department of Clinical Epidemiology at Aarhus University Hospital participates in the International Diabetic Neuropathy Consortium (IDNC) research programme, which is supported by a Novo Nordisk Foundation Challenge programme grant (grant no. NNF14SA000 6). TL owns shares in Novo Nordisk A/S. Department of Clinical Epidemiology is involved in studies with funding from various companies as research grants to (and administered by) Aarhus University. None of these studies are related to the present study.
Prior Presentation: the study has not been presented elsewhere.
Ethics approval: Not needed for purely registry-based studies in Denmark.
Patient involvement
Patients were not involved in posing the research question, choosing the outcome measures, or in the design or implementation of the study. There are no plans to involve patients in the dissemination of the results.
Data sharing: Due to restrictions related to Danish law and protecting patient privacy, the combined set of data as used in this study can only be made available through a trusted third party, Statistics Denmark. This state organisation holds the data used for this study. University-based Danish scientific organisations can be authorized to work with data within Statistics Denmark and such organisations can provide access to individual scientists inside and outside of Denmark. Requests for data may be directed to Statistics Denmark: forskningsservice@dst.dk or +45 39 17 31 32.
Transparency: The senior author, RWT, affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Copyright: The corresponding author has the right to grant on behalf of all authors and does grant on behalf of all authors, to the Publishers and its licensees in perpetuity, in all forms, formats and media (whether known now or created in the future), to i) publish, reproduce, distribute, display and store the Contribution, ii) translate the Contribution into other languages, create adaptations, reprints, include within collections and create summaries, extracts and/or, abstracts of the Contribution, iii) create any other derivative work(s) based on the Contribution, iv) exploit all subsidiary rights in the Contribution, v) include electronic links from the Contribution to third party material wherever it may be located; and, vi) license any third party to do any or all of the above.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2021.100291.
Appendix. Supplementary materials
Table S1. Sex, age, comorbidity, and HbA1c of comparison cohort, by calendar period of diagnosis.
Figure S2. Age standardized incidence rates for men and women.
Table S3: Mortality rates and rate ratios comparing diabetes patients and comparators within diagnosis periods.
Figure S4. Proportion of patients diagnosed with type 2 diabetes each calendar year by baseline HbA1c measurement.
Figure S5. All-cause mortality rates for the type 2 diabetes cohort and age- and sex-matched comparators with 95% confidence intervals by calendar year of diagnosis.
Figure S6. Cumulative unadjusted all-cause mortality (%) by calendar year of diagnosis.
Figure S7. Proportion of patients receiving HbA1c, 2-hour oral glucose tolerance testing, or fasting plasma glucose measurement one year prior to diagnosis by calendar year of diagnosis. Total number of patients that had any of the diagnostic tests by calendar year of diagnosis
Figure S8. Annual percentage change in type 2 diabetes incidence and mortality.
Figure S9. Sensitivity analysis: Age standardized incidence rates for men and women
Table S10. Sensitivity analysis: Mortality rate and mortality rate ratios over time for T2D patients and age- and sex-matched comparators.
Figure S11. Sensitivity analysis: Mortality rates and rate ratios comparing diabetes patients and comparators within diagnosis periods.
Figure S12 mortality rate ratios over time for T2D patients by group using 1995 as reference.
References
- 1.WHO . 2016. Global Report on Diabetes.https://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf;jsessionid=F57B0CDDF4D02AA4BCF8F920D4B3AEBB?sequence=1 available at. accessed 2019-12-23. [Google Scholar]
- 2.Jensen HAR, Thygesen LC, Davidsen M. 2017. Sygdomsudviklingen i Danmark fremskrevet til 2030 - KOL og type 2-diabetes.https://sum.dk/Aktuelt/Nyheder/Sygehusvaesen/2017/Juni/∼/media/Fremskrivningsrapport.ashx available at. accessed 2019-12-23. [Google Scholar]
- 3.Saeedi P, Petersohn I, Salpea P, et al. IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019 Nov;157 doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organisation . 2011. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus.https://www.ncbi.nlm.nih.gov/books/NBK304267 available at. /accessed 2020-03-23. [Google Scholar]
- 5.Mccane DR, Hanson RL, Charles MA, et al. Comparison of tests for glycated haemoglobin and fasting and two hour plasma glucose concentrations as diagnostic methods for diabetes. BMJ. 1994;308:1323. doi: 10.1136/bmj.308.6940.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauritzen T, Sandbaek A, Skriver MV, Borch-Johnsen K. HbA1c and cardiovascular risk score identify people who may benefit from preventive interventions: A 7 year follow-up of a high-risk screening programme for diabetes in primary care (ADDITION), Denmark. Diabetologia. 2011;54:1318–1326. doi: 10.1007/s00125-011-2077-9. [DOI] [PubMed] [Google Scholar]
- 7.Gyberg V, De Bacquer D, Kotseva K, et al. Screening for dysglycaemia in patients with coronary artery disease as reflected by fasting glucose, oral glucose tolerance test, and HbA1c: A report from EUROASPIRE IV - A survey from the European Society of Cardiology. Eur Heart J. 2015;36:1171–1177c. doi: 10.1093/eurheartj/ehv008. [DOI] [PubMed] [Google Scholar]
- 8.Danish National Board of Health . 2012. Note regarding the use of HbA1c for diagnostic testing in Denmark.https://www.sst.dk/da/∼/∼/media/2A3178A6D31B428FA888E39AA46B0B4E.ashx available at. (accessed March 4, 2020) [Google Scholar]
- 9.Knudsen JS, Hulman A, Rønn PF, Lauritzen T, Sørensen HT, Witte DR. Trends in HbA 1c and LDL Cholesterol in Patients With Type 2 Diabetes Receiving First-Time Treatment in Northern Sequential Cross-Sectional Analysis. Diabetes Care. 2019:e1–e3. doi: 10.2337/dc19-0527. [DOI] [PubMed] [Google Scholar]
- 10.Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care. 2011 May;34 Suppl 2(Suppl 2):S184–S190. doi: 10.2337/dc11-s216. PMID: 21525453; PMCID: PMC3632159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magliano DJ, Islam RM, Barr ELM, et al. Trends in incidence of total or type 2 diabetes: Systematic review. BMJ. 2019;366:1–12. doi: 10.1136/bmj.l5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selvin E, Ali MK. Declines in the incidence of diabetes in the U.S.-real progress or artifact? Diabetes Care. 2017;40:1139–1143. doi: 10.2337/dc16-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jørgensen ME, Ellervik C, Ekholm O, Johansen NB, Carstensen B. Estimates of prediabetes and undiagnosed type 2 diabetes in Denmark: The end of an epidemic or a diagnostic artefact? Scand J Public Health. 2018:1–7. doi: 10.1177/1403494818799606. [DOI] [PubMed] [Google Scholar]
- 14.Magliano DJ, Chen L, Islam RM. Trends in the incidence of diagnosed diabetes: a multicountry analysis of aggregate data from 22 million diagnoses in high-income and middle-income settings. Lancet Diabetes Endocrinol. 2021 Apr;9(4):203–211. doi: 10.1016/S2213-8587(20)30402-2. Epub 2021 Feb 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruiz PLD, Stene LC, Bakken IJ, Håberg SE, Birkeland KI, Gulseth HL. Decreasing incidence of pharmacologically and non-pharmacologically treated type 2 diabetes in Norway: a nationwide study. Diabetologia. 2018;61:2310–2318. doi: 10.1007/s00125-018-4681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N Engl J Med. 2017;376:1419–1429. doi: 10.1056/NEJMoa1610187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norhammar A, Bodegård J, Nyström T, Thuresson M, Eriksson JW, Nathanson D. Incidence, prevalence and mortality of type 2 diabetes requiring glucose-lowering treatment, and associated risks of cardiovascular complications: a nationwide study in Sweden, 2006–2013. Diabetologia. 2016;59:1692–1701. doi: 10.1007/s00125-016-3971-y. [DOI] [PubMed] [Google Scholar]
- 18.Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376:1407–1418. doi: 10.1056/NEJMoa1608664. [DOI] [PubMed] [Google Scholar]
- 19.Gregg EW, Cheng YJ, Srinivasan M, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet. 2018;391:2430–2440. doi: 10.1016/S0140-6736(18)30314-3. [DOI] [PubMed] [Google Scholar]
- 20.Carstensen B, Rønn PF, Jørgensen ME. Components of diabetes prevalence in Denmark 1996-2016 and future trends until 2030. BMJ Open Diabetes Res Care. 2020 Aug;8(1) doi: 10.1136/bmjdrc-2019-001064. PMID: 32784246; PMCID: PMC7418686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591. doi: 10.2147/CLEP.S179083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The generation of the national diabetes register (Danish) 1996. Available at http://www.ssi.dk/∼/media/Indhold/DK%20-%20dansk/Sundhedsdata%20og%20it/NSF/Registre/Diabetesregisteret/Sådan%20dannes%20Det%20Nationale%20Diabetesregister.ashx. accessed 2020-03-23
- 23.O'Connell RL, Lim LL-Y. Utility of the Charlson Comorbidity Index Computed from Routinely Collected Hospital Discharge Diagnosis Codes. Methods Arch. 2000;39:7–11. [PubMed] [Google Scholar]
- 24.Knudsen JS, Thomsen RW, Pottegård A, Knop FK, Sørensen HT. Clinical characteristics and glucose-lowering drug utilization among patients initiating liraglutide in Denmark: a routine clinical care prescription study. J Diabetes. 2019;11:690–694. doi: 10.1111/1753-0407.12919. [DOI] [PubMed] [Google Scholar]
- 25.Fox CS, Pencina MJ, Meigs JB, Vasan RS, Levitzky YS, D'Agostino RB. Trends in the incidence of type 2 diabetes mellitus from the 1970s to the 1990s: The Framingham Heart Study. Circulation. 2006;113:2914–2918. doi: 10.1161/CIRCULATIONAHA.106.613828. [DOI] [PubMed] [Google Scholar]
- 26.Forssas E, Arffman M, Koskinen S, Reunanen A, Keskimäki I. Socioeconomic differences in mortality among diabetic people in Finland. Scand J Public Health. 2010;38:691–698. doi: 10.1177/1403494810376427. [DOI] [PubMed] [Google Scholar]
- 27.Green A, Sortsø C, Jensen PB, Emneus M. Incidence, morbidity, mortality, and prevalence of diabetes in Denmark, 2000–2011: Results from the Diabetes Impact Study 2013. Clin Epidemiol. 2015;7:421–430. doi: 10.2147/CLEP.S88577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carstensen B, Rønn PF, Jørgensen ME. Prevalence, incidence and mortality of type 1 and type 2 diabetes in Denmark 1996-2016. BMJ Open Diabetes Res Care. 2020 May;8(1) doi: 10.1136/bmjdrc-2019-001071. PMID: 32475839; PMCID: PMC7265004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carstensen B, Rønn PF, Jørgensen ME. Lifetime risk and years lost to type 1 and type 2 diabetes in Denmark, 1996-2016. BMJ Open Diabetes Res Care. 2021 Mar;9(1) doi: 10.1136/bmjdrc-2019-001065. PMID: 33653710; PMCID: PMC7929801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Read SH, Kerssens JJ, McAllister DA, et al. Trends in type 2 diabetes incidence and mortality in Scotland between 2004 and 2013. Diabetologia. 2016;59:2106–2113. doi: 10.1007/s00125-016-4054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zghebi SS, Steinke DT, Carr MJ, Rutter MK, Emsley RA, Ashcroft DM. Examining trends in type 2 diabetes incidence, prevalence and mortality in the UK between 2004 and 2014. Diabetes, Obes Metab. 2017;19:1537–1545. doi: 10.1111/dom.12964. [DOI] [PubMed] [Google Scholar]
- 32.de Sousa-Uva M, Antunes L, Nunes B, et al. Trends in diabetes incidence from 1992 to 2015 and projections for 2024: A Portuguese General Practitioner's Network study. Prim Care Diabetes. 2016;10:329–333. doi: 10.1016/j.pcd.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Harding JL, Shaw JE, Peeters A, Davidson S, Magliano DJ. Age-Specific Trends From 2000-2011 in All-Cause and Cause-Specific Mortality in Type 1 and Type 2 Diabetes: A Cohort Study of More Than One Million People. Diabetes Care. 2016;39:1018–1026. doi: 10.2337/dc15-2308. [DOI] [PubMed] [Google Scholar]
- 34.Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA. 2014;312:1218–1226. doi: 10.1001/jama.2014.11494. [DOI] [PubMed] [Google Scholar]
- 35.American Diabetes Association Introduction: Standards of Medical Care in Diabetes - 2019. Diabetes Care. 2019;42:S1–S2. doi: 10.2337/dc19-Sint01. [DOI] [PubMed] [Google Scholar]
- 36.The ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 37.Green A, Sortsø C, Jensen PB, Emneus M. Validation of the danish national diabetes register. Clin Epidemiol. 2014;7:5–15. doi: 10.2147/CLEP.S72768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Analyses based on the reconstructed Danish Diabetes Register Report available at http://bendixcarstensen.com/DMreg/Ana2016.pdf. Accessed 30.09.2021
- 39.Shahim B, De Bacquer D, De Backer G, et al. The prognostic value of fasting plasma glucose, two-hour postload glucose, and HbA1c in patients with coronary artery disease: A report from EUROASPIRE IV: A survey from the european society of cardiology. Diabetes Care. 2017;40:1233–1240. doi: 10.2337/dc17-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meigs JB, Nathan DM, D'Agostino RB, Sr., Wilson PW. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002;25:1845–1850. doi: 10.2337/diacare.25.10.1845. [DOI] [PubMed] [Google Scholar]
- 41.Qiao Q, Dekker JM, de Vegt F, Nijpels G, Nissinen A, Stehouwer CD, Bouter LM, Heine RJ, Tuomilehto J. Two prospective studies found that elevated 2-hr glucose predicted male mortality independent of fasting glucose and HbA1c. J Clin Epidemiol. 2004;57:590–596. doi: 10.1016/j.jclinepi.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Cai X, Zhang Y, Li M, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: Updated meta-analysis. BMJ. 2020;370:m2297. doi: 10.1136/bmj.m2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen I, Nicolaisen SK, Ricciardi F, et al. Impact of Being Eligible for Type 2 Diabetes Treatment on All-Cause Mortality and Cardiovascular Events: Regression Discontinuity Design Study. Clin Epidemiol. 2020 Jun 3;12:569–577. doi: 10.2147/CLEP.S251704. PMID: 32606982; PMCID: PMC7294562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.SCORE2 working group and ESC Cardiovascular risk collaboration SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. European Heart Journal. 2021;Volume 42(Issue 25):2439–2454. doi: 10.1093/eurheartj/ehab309. 1 July. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Sex, age, comorbidity, and HbA1c of comparison cohort, by calendar period of diagnosis.
Figure S2. Age standardized incidence rates for men and women.
Table S3: Mortality rates and rate ratios comparing diabetes patients and comparators within diagnosis periods.
Figure S4. Proportion of patients diagnosed with type 2 diabetes each calendar year by baseline HbA1c measurement.
Figure S5. All-cause mortality rates for the type 2 diabetes cohort and age- and sex-matched comparators with 95% confidence intervals by calendar year of diagnosis.
Figure S6. Cumulative unadjusted all-cause mortality (%) by calendar year of diagnosis.
Figure S7. Proportion of patients receiving HbA1c, 2-hour oral glucose tolerance testing, or fasting plasma glucose measurement one year prior to diagnosis by calendar year of diagnosis. Total number of patients that had any of the diagnostic tests by calendar year of diagnosis
Figure S8. Annual percentage change in type 2 diabetes incidence and mortality.
Figure S9. Sensitivity analysis: Age standardized incidence rates for men and women
Table S10. Sensitivity analysis: Mortality rate and mortality rate ratios over time for T2D patients and age- and sex-matched comparators.
Figure S11. Sensitivity analysis: Mortality rates and rate ratios comparing diabetes patients and comparators within diagnosis periods.
Figure S12 mortality rate ratios over time for T2D patients by group using 1995 as reference.