Figure 1.
Primary assay development
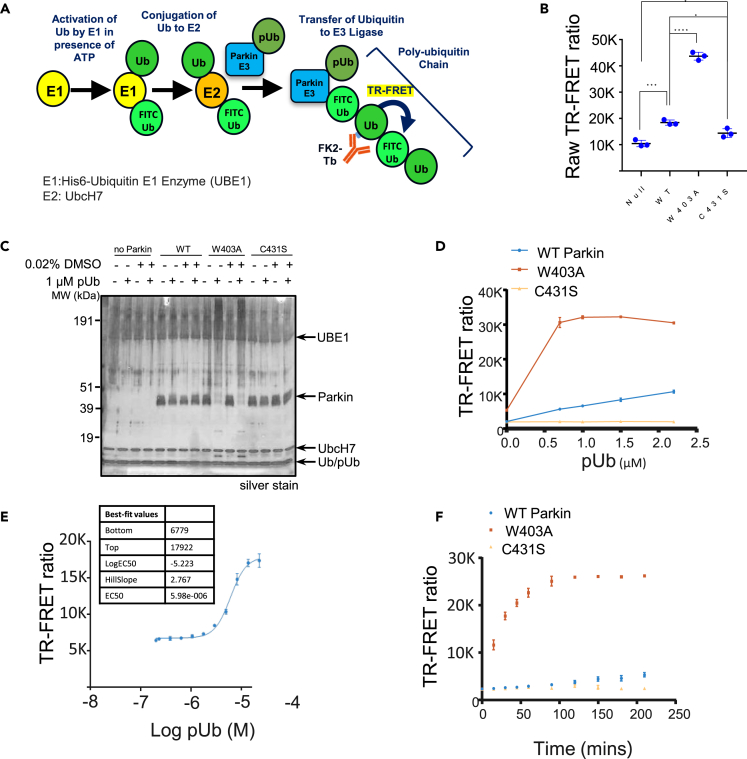
(A) Schematic of the biochemical assay including detection by an FK2 antibody, which recognizes polyubiquitin and provides an FRET signal to the FITC-labeled ubiquitin. The components of the reaction are depicted with circles.
(B) Comparison of the FRET ratio of the WT-, W403A- and C431S-Parkin after 1 h incubation.
(C) Silver stain indicating that W403A-Parkin was more active than WT-Parkin in the presence of 1 μM pUb, as indicated by the disappearance of the Parkin band and the appearance of a high-molecular weight smear.
(D) Dependency of WT- (blue) and W403A-Parkin (red) activity on pUb concentration. The C431S-Parkin (yellow) is inactive at all pUb concentrations.
(E) Titration of pUb in TR-FRET assay with WT-Parkin (blue) at a 3 h reaction time and calculation of Ec50. Each data point represents 2 technical replicates.
(F) At a given time (i.e., 180 min), we can enable a large window between WT-Parkin (blue) and the W403A-Parkin positive control (red). Each data point represents 2 technical replicates. Statistical test performed: ANOVA with Dunnett’s MCT versus WT ∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. For panel B, error bars represent standard deviation with n = 3 technical replicates. See also Figure S1.