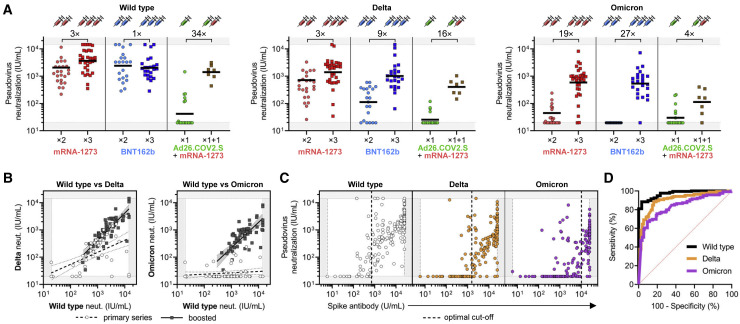
Figure 3.
Cross-reactivity of neutralizing antibody response is increased by an additional dose (“booster”) of mRNA vaccine relative to primary vaccination series and can be predicted by anti-spike antibody levels
(A) Neutralization titers (in WHO IU/mL) of wild-type (WT; left panel), Delta (middle panel), and Omicron (right panel) SARS-CoV-2 pseudoviruses were analyzed for infection-naive participants that were recently vaccinated with primary series or booster (<3 months). Recently vaccinated individuals received mRNA-1273 (×2), BNT162b (×2), or Ad26.COV2.S (×1), and boosted individuals received a homologous booster of mRNA-1273 (×3), or BNT162b (×3), or a cross-over booster of mRNA-1273 for Ad26.COV2.S vaccinees (×1 + 1). Fold-increase in geometric mean neutralization titer of boosted versus non-boosted individuals is shown as a number with “×” symbol. This analysis is based on experimental data depicted in Figure 2C but excludes participants with prior SARS-CoV-2 infection, distant vaccination (>6 months), and/or cross-over between mRNA-1273 and BNT162b to understand differences in neutralizing responses soon after primary vaccination series versus boosting. The single Ad26.COV2.S vaccinee that received a homologous boost with Ad26.COV2.S was also excluded from this analysis. Dark horizontal lines for each group denote geometric mean titer.
(B) Aggregate data from study participants in (A) that recently received primary vaccination series (“primary series”; white circles) or were recently boosted (“boosted”; dark gray squares) were used for linear regression analysis of wild type versus Delta (left panel) or wild type versus Omicron (right panel) pseudovirus neutralization. Wild-type neutralization titers correlated with Delta neutralization in “primary series” individuals (R2 = 0.35; slope = 0.44; p < 0.0001) and even more strongly in “boosted” individuals (R2 = 0.68; slope = 1.00; p < 0.0001). Wild-type neutralization titers showed no significant relationship with Omicron neutralization in “primary series” individuals (R2 = 0.03; slope = 0.05; p = 0.16); however, “boosted” individuals showed a significant correlation with Omicron neutralization titers (R2 = 0.56; slope = 0.94; p < 0.0001).
(C) Anti-SARS-CoV-2 spike antibodies levels (measured by an EUA-approved clinical diagnostic test) of all vaccinees were plotted against neutralization of wild-type (left panel; white circles), Delta (middle panel; orange circles), and Omicron (right panel; purple circles) SARS-CoV-2 pseudoviruses. Optimal spike antibody cut-offs for predicting positive neutralization were determined by ROC analyses in (D) and are indicated with a vertical dashed line.
(D) Receiver operating characteristic (ROC) analyses assessing the ability of spike antibody levels to predict neutralization of wild-type (black line), Delta (orange line), and Omicron (purple line) pseudoviruses. Positive neutralization was defined as >33 IU/mL (previously defined with a cohort of 1,200 pre-pandemic samples (Garcia-Beltran et al., 2021b) and converted to WHO IU/mL). Area under curve (AUC) for wild type was 0.97, Delta was 0.91, and Omicron was 0.84, with p < 0.0001 for all three. Optimal cut-offs that maximized sensitivity (Se) and specificity (Sp) were determined using the “Se + Sp” method, and were as follows: for wild type, optimal cut-off of 711 U/mL achieved 88.4% Se and 96.7% Sp; for Delta, optimal cut-off of 1,591 U/mL achieved 88.4% Se and 83.8% Sp; and for Omicron variant, optimal cut-off of 10,300 achieved 67.2% Se and 90.6% Sp. These optimal cut-off values are plotted as a vertical dashed line in (C).