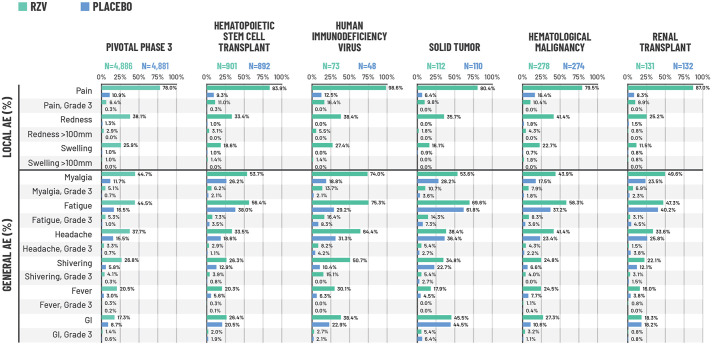
Figure 1.
Percentage of participants reporting solicited local and general AEs during the 7-day post-vaccination period in the combined analysis of the two pivotal phase-3 trials (ZOE-50 and ZOE-70)21,22 and studies of immunocompromised populations.
*Each population was evaluated in a different study. Pivotal phase-3 trials: data from the pooled safety analysis of the ZOE-50 and ZOE-70 trials in adults ⩾50 years of age. For the pivotal phase-3 ZOE trials, N corresponds to a sub-cohort of participants who were asked to complete a 7-day diary card recording solicited AEs and who received ⩾1 vaccine dose. This sub-cohort consisted of 4886 participants in the RZV group and 4881 participants in the placebo group; 4884 participants in the RZV group and 4880 participants in the placebo group returned their diary cards reporting local events, while 4876 participants in the RZV group and 4881 participants in the placebo group returned their diary cards reporting general events. For the studies in immunocompromised populations, all participants in the TVC were asked to complete a diary card; the N values shown correspond to the number who returned a diary card and received ⩾1 vaccine dose. AEs were recorded for 7 days (on the day of vaccination and 6 days thereafter). Grade-3 pain and general solicited AEs: prevented normal, everyday activities. Grade-3 redness and swelling: >100 mm. Fever: temperature ⩾37.5°C. Grade-3 fever: temperature >39.0°C.
AE, adverse event; GI, gastrointestinal; HSCT, hematopoietic stem cell transplant; RZV, recombinant zoster vaccine; TVC, total vaccinated cohort.