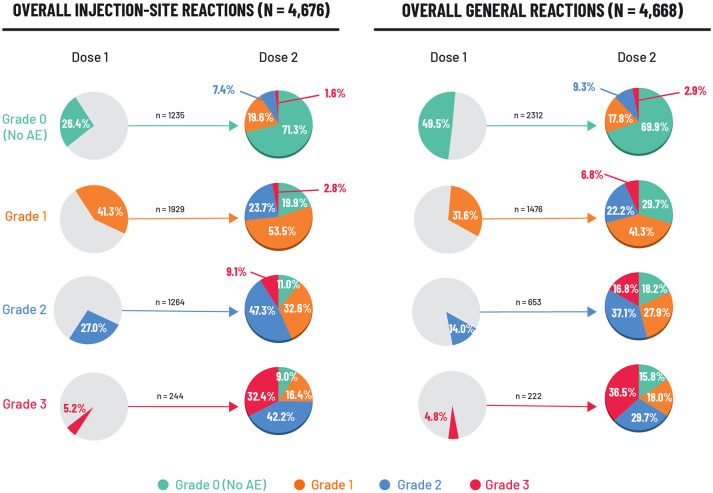
Figure 2.
Intensity (grade) of solicited local and general AEs reported after the second RZV dose in relation to the first dose (ZOE-50 and ZOE-70). 25
Data are reported for the reactogenicity sub-cohort which comprised participants who completed a diary card recording AEs after vaccination. AEs were recorded for 7 days (on the day of vaccination and 6 days thereafter). N: number of RZV vaccinees with both doses administered and corresponding event intensity after Dose 1 and/or after Dose 2. n%: number and percentage of RZV vaccinees with events at a specific grade. There were nine events (four injection site and five general events) with missing grading after Dose 1 and 6 events (three injection site and three general events) with missing grading after Dose 2. Injection site events included: pain at the injection site, redness at the injection site, and swelling at the injection site. General events included: fatigue, gastrointestinal symptoms (nausea, vomiting, diarrhea and/or abdominal pain), headache, myalgia, shiver, and fever.
AE, adverse event; RZV, recombinant zoster vaccine.