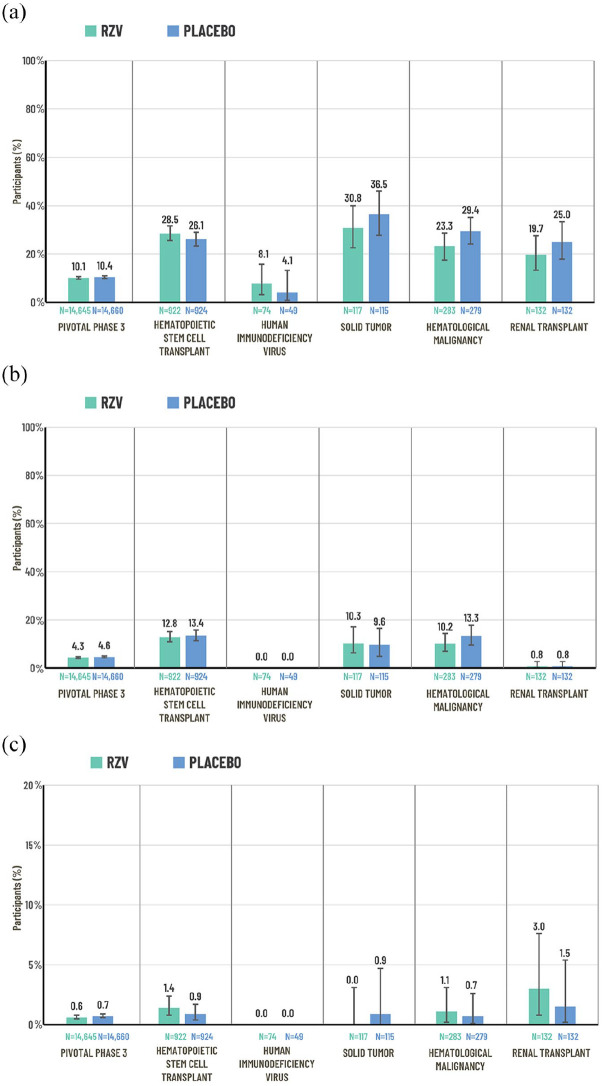
Figure 3.
Percentage of participants reporting SAEs, fatal AEs and pIMDs in the combined analysis of the two pivotal Phase-3 trials (ZOE-50 and ZOE-70)21,22 and studies of immunocompromised populations:12–14,16–19 (a) SAEsa, (b) fatal AEs,a and (c) pIMDsa.
Pivotal Phase-3 trials: data from the pooled safety analysis of the ZOE-50 and ZOE-70 trials in adults ⩾50 years of age. Data are reported for the total vaccinated cohort which comprised participants who received at least one vaccine dose. SAEs and pIMDs were recorded over the following study periods: ZOE and HSCT: from first vaccination up to 1 year after last vaccination; other studies: from first vaccination until study end (approximately 12 months after the last scheduled dose). Fatal AEs were recorded from first vaccination until study end (approximately 12 months after the last scheduled dose for all studies except HSCT and ZOE; the median duration of the safety follow-up was 29 months for the HSCT trial and 4.4 years for the pooled safety analysis of the ZOE trials). Error bars represent 95% CIs.
AE, adverse event; CI, confidence interval; N, number of patients in the total vaccinated cohort per group; pIMD, potential immune-mediated disease; RZV, recombinant zoster vaccine; SAE, serious adverse event; TVC, total vaccinated cohort.
aEach population was evaluated in a different study.