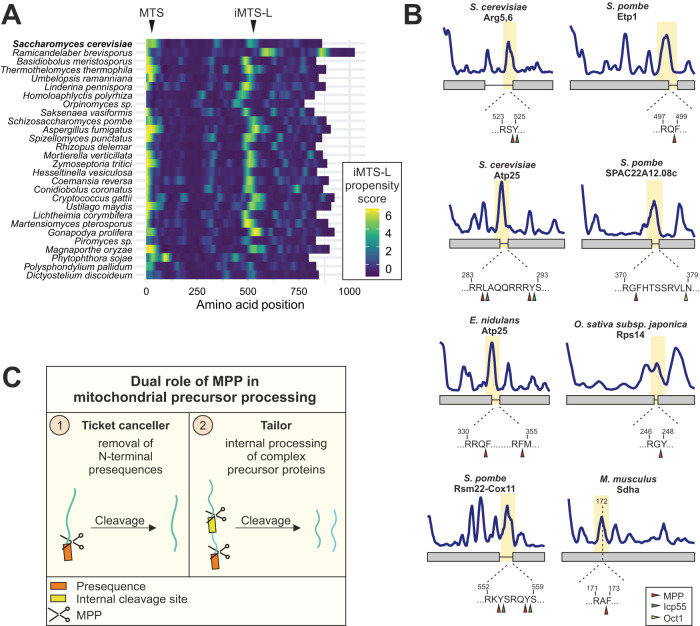
FIGURE 6:
MPP not only functions as a presequence peptidase, but also has a conserved processing activity at internal cleavage sites of composite precursor proteins. (A) The iMTS-L at which MPP cleaves the Arg5,6 precursor in S. cerevisiae is conserved among species that encode Arg5,6 as fusion protein. Shown are iMTS-L propensity profiles along the sequence of Arg5,6 fusion protein homologues. (B) Previously described mitochondrial polyproteins from different species harbor an iMTS-L at the position of the junction between the fused polypeptides. Arrowheads indicate potential cleavage sites for MPP (red) and the downstream processing peptidases Icp55 (green) or Oct1 (yellow) as predicted by MitoFates. Sdha is no polyprotein, but an alternative N-terminus (dashed line) was recently identified, presumably the result of a posttranslational cleavage (Calvo et al., 2017). (C) In addition to its canonical role in presequence removal from mitochondrial precursor proteins, MPP has a second conserved function. It recognizes internal cleavage sites and processes complex precursor proteins into separate polypeptides.