Abstract
Background
To date, studies investigating the inflammatory bowel disease (IBD) patient experience with coronavirus disease 2019 (COVID-19) have consistently reported that the observed rate of COVID-19 within this population is similar to the general population. Limited research has suggested that corticosteroid use in the IBD population may be associated with worse COVID-19 outcomes, but it is still yet to be determined if specific IBD-related clinical factors are associated with worse outcomes. Our goal was to describe clinical COVID-19 outcomes for IBD patients and to identify the clinical factors that may be associated with worse outcomes.
Methods
In this retrospective study, we utilized the inpatient database within the largest hospital network in the New York City Metropolitan area to identify all IBD patients with confirmed COVID-19.
Results
Of 83 IBD/COVID-19 patients presenting to a hospital network emergency room, 56 were hospitalized. Overall, 19.6% of hospitalized IBD patients died, compared with 22.2% of all hospital system COVID-19 patients during the time period. There was no association between pre-admission corticosteroid use or biologic treatment with a severe course of COVID-19.
Conclusions
In contrast to some prior reports, we did not observe an association of pre-admission corticosteroid use and adverse outcomes. While the mortality rate was high for IBD/COVID-19 patients, it was not greater than that for hospitalized COVID-19 patients generally. Though our results are encouraging, we continue to support the recommendations of the leading gastrointestinal and IBD societies to regard our patients as “at risk”, and to observe caution in their care.
Keywords: Biologic therapy, Colitis, COVID-19, IBD, SARS-CoV-2
Introduction
The first cases of a novel coronavirus infection were reported in Wuhan, China in December of 2019 [1]. Since that time the virus has spread to all continents except Antarctica, with the World Health Organization declaring a global pandemic on March 11, 2020 [2]. Symptomatic patients typically develop fever, cough, and shortness of breath. A significant minority progress to severe lung injury requiring the need for mechanical ventilation, with an attendant high mortality rate [3-5]. Since its identification, the virus has been assigned the formal nomenclature of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with the associated clinical illness designated as 2019 novel coronavirus (2019-nCoV) or coronavirus disease 2019 (COVID-19) [6]. The gastrointestinal (GI) tract is believed to be a particularly important source of access to viral infection given the high expression of angiotensin-converting enzyme 2 (ACE2) receptor, which is a key binding site for the coronavirus [7-10]. Viral binding and subsequent cell entry is accomplished via the spike protein on the viral surface. Once the viral RNA is incorporated into the host genome, the virus is then assembled and secreted along with inflammatory cytokines which may explain the various GI symptoms (i.e., nausea, vomiting, abdominal pain, and diarrhea) frequently observed in COVID-19 infection [11].
Since the early outbreak, those at highest risk were noted to be the elderly and those with preexisting medical conditions such as cardiovascular, respiratory, endocrine and oncologic disease [12, 13]. Additionally, since the first days of the pandemic, there has been particular concern for infection among vulnerable populations, including those with immune-mediated diseases and those requiring immunosuppressive therapies for treatment. Within the international GI community, attention rapidly focused on those with inflammatory bowel disease (IBD). Almost immediately, several leading national and international GI and IBD societies published position papers and guidelines advising how best to protect and manage IBD patients in the face of the public health crisis [14-16]. While not broadly advocating for a suspension of IBD therapies, guidelines were provided for prevention of infection as well as preferred management strategies for IBD during the pandemic.
In the following months, a rapid proliferation of case reports, case series, database studies, and international registry data has sought to investigate the IBD/COVID-19 experience. Overall, these reports have observed rates of COVID-19 among IBD patients to be similar to the general population [17-24]. Currently the largest IBD/COVID-19 database, the Surveillance Epidemiology of Coronavirus Under Research Exclusion for IBD (SECURE-IBD), has investigated factors associated with poor IBD/COVID-19 outcomes [25]. While acknowledging the potential of reporting bias, the authors observed no increased risk of adverse outcomes related to immune or biologic therapy, but did find an increased mortality with corticosteroid use.
For most of the single institution and regional case series, the numbers have been too small for an analysis of outcomes of COVID-19 based on specific IBD treatment exposures. The largest systematic study by Bezzio et al analyzed outcomes from 79 patients across 24 Italian IBD referral centers, and failed to find an association between IBD medication use and adverse outcomes [26]. Among the total, only 22 patients required hospitalization. Singh et al utilized a large international patient database to identify 232 patients with IBD and COVID-19, and again did not observe adverse outcomes associated with immune or biologic therapies, but did note worse outcomes with corticosteroid use [27]. For the 56 patients who were hospitalized, there was no specific sub-analysis of outcome variables in this sickest population, nor could the methodology fully validate the IBD or COVID-19 diagnosis by direct chart review.
The Northwell Health system is the largest health care provider in the New York City metropolitan region, including four tertiary care teaching institutions and another 20 community hospitals serving 11 million persons in New York City, Long Island, and Westchester County. As the “epicenter of the epicenter” of the initial United States COVID-19 outbreak, the data from this hospital system were the first to provide a large scale comprehensive report of outcomes from over 5,700 hospitalized patients [28]. Our goal was to query the Northwell Health inpatient database, identify IBD patients with confirmed COVID-19, describe their outcomes and provide analysis of clinical factors influencing these outcomes.
Materials and Methods
We conducted a retrospective study of adult IBD patients with COVID-19 who required hospitalization between January 1, 2020 and October 6, 2020 across the Northwell Health system. This study was approved by the Northwell Health Institutional Review Board. All procedures performed in studies involving human subjects were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Data were collected from the enterprise health record (Inpatient: Sunrise Clinical Manager, Outpatient: Allscripts) reporting database. Cases were identified by ICD-10 codes for IBD (K50.xx, K51.xx, and K52.3, for Crohn’s disease (CD), ulcerative colitis (UC) and indeterminate colitis, respectively), with COVID-19 defined by ICD-10 code (U07.1) and a COVID-19 polymerase chain reaction (PCR) positive result. Following initial patient identification, each identified record was individually reviewed to confirm both the IBD status and the COVID-19 diagnosis. Data collected included patient demographics, presenting complaints, outpatient medications, inpatient treatments, hospital course, and mortality. An adverse outcome was defined by intensive care unit (ICU) admission, ventilator use, acute kidney injury (AKI), or death. A composite adverse outcome variable included at least one of the following: ICU admission, ventilator use, or death. Chi-square and Fisher’s exact tests were utilized to assess potential risk factors for adverse outcomes.
Results
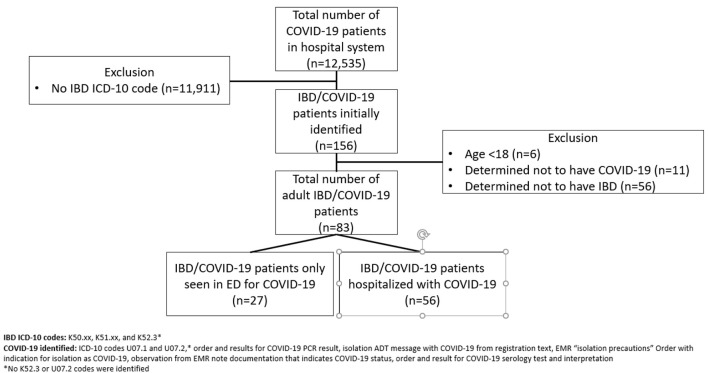
Of 12,535 COVID-19 patients evaluated within the hospital network, 156 patients met the initial ICD-10 code search criteria (Fig. 1). Six patients were immediately excluded for being minors, as the analyses presented here were limited to adult IBD patients. Following chart review, a remaining 83 adult patients with confirmed IBD and COVID-19 were identified, of whom 56 required hospitalization. Over half of the hospitalized cohort had CD (51.8%), 28 (50%) were female, 38 identified as white (79.2%), with a mean age of 60.7 years. Hypertension was the most common medical comorbidity (48.2%), followed by either type 1 or 2 diabetes (30.3%). Over half of the patients were receiving medical management for their IBD at the time of hospital admission (51.8%). Common IBD therapies that patients were receiving include mesalamine (30.4%), prednisone (17.9%), and biologics (12.5%) (Table 1).
Figure 1.
Flowchart of IBD with COVID-19 patient identification. IBD: inflammatory bowel disease; COVID-19: coronavirus disease 2019.
Table 1. Demographics, Clinical Characteristics, and IBD Medications of All IBD/COVID-19 Patients and Patients Separated Into ED Visit Only and Hospitalized (n = 83).
| All IBD/COVID-19 (n = 83) |
IBD/COVID-19 patients with ED visit only (n = 27) |
IBD/COVID-19 patients hospitalized (n = 56) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | # | % | n | # | % | n | # | % | |
| Demographics | |||||||||
| Sex | 83 | 27 | 56 | ||||||
| Female | 41 | 49.4 | 13 | 48.2 | 28 | 50.0 | |||
| Male | 42 | 50.6 | 14 | 51.9 | 28 | 50.0 | |||
| Age, mean (SD), years | 83 | 56.9 | 18.5 | 27 | 49.0 | 12.7 | 56 | 60.7 | 19.7 |
| Race | 70 | 22 | 48 | ||||||
| White | 52 | 74.3 | 14 | 63.6 | 38 | 79.2 | |||
| Black or African American | 9 | 12.9 | 4 | 18.2 | 5 | 10.4 | |||
| Asian | 2 | 2.9 | 1 | 4.5 | 1 | 2.1 | |||
| American Indian/native Alaskan | 1 | 1.4 | 1 | 4.5 | 0 | 0 | |||
| Multiracial | 6 | 8.6 | 2 | 9.1 | 4 | 8.3 | |||
| Ethnicity | 61 | 21 | 40 | ||||||
| Hispanic/Latino | 7 | 11.5 | 4 | 19.0 | 3 | 7.5 | |||
| Not Hispanic/Latino | 54 | 88.5 | 17 | 81.0 | 37 | 92.5 | |||
| Health characteristics | |||||||||
| BMIa | 66 | 16 | 50 | ||||||
| < 18.5 (underweight) | 2 | 3.0 | 0 | 0 | 2 | 4.0 | |||
| 18.5 - 24.9 (normal weight) | 22 | 33.3 | 4 | 25.0 | 18 | 36.0 | |||
| 25.0 - 29.9 (overweight) | 16 | 24.2 | 5 | 31.3 | 11 | 22.0 | |||
| 30+ (obese) | 26 | 39.4 | 7 | 43.8 | 19 | 38.0 | |||
| Smoking status (cigarettes) | 72 | 23 | 49 | ||||||
| Current smoker | 2 | 2.8 | 1 | 4.3 | 1 | 2.0 | |||
| Prior smoker | 14 | 19.4 | 2 | 8.7 | 12 | 24.5 | |||
| Never smoker | 56 | 77.8 | 20 | 87.0 | 36 | 73.5 | |||
| IBD diagnosis | 83 | 27 | 56 | ||||||
| CD | 46 | 55.4 | 17 | 63.0 | 29 | 51.8 | |||
| UC | 35 | 42.2 | 10 | 37.0 | 25 | 44.6 | |||
| Indeterminate colitis | 2 | 2.4 | 0 | 0 | 2 | 3.6 | |||
| Comorbidities | 83 | 27 | 56 | ||||||
| AIDS | 1 | 1.2 | 0 | 0 | 1 | 1.8 | |||
| Anxiety | 11 | 13.3 | 0 | 0 | 11 | 19.6 | |||
| Asthma | 4 | 4.8 | 1 | 3.7 | 3 | 5.4 | |||
| Bipolar disorder | 2 | 2.4 | 0 | 0 | 2 | 3.6 | |||
| Cancer (current)b | 3 | 3.6 | 0 | 0 | 3 | 5.4 | |||
| Colon cancerc | 2 | 2.4 | 0 | 0 | 2 | 3.6 | |||
| Pancreatic cancer | 1 | 1.2 | 0 | 0 | 1 | 1.8 | |||
| Cancer (prior)b | 9 | 10.8 | 5 | 18.5 | 4 | 7.1 | |||
| Breast cancer | 2 | 2.4 | 2 | 7.4 | 0 | 0 | |||
| Colon cancer | 2 | 2.4 | 0 | 0 | 2 | 3.6 | |||
| Lung cancer | 1 | 1.2 | 0 | 0 | 1 | 1.8 | |||
| Melanoma | 1 | 1.2 | 1 | 3.7 | 0 | 0 | |||
| Non-Hodgkin’s lymphoma | 1 | 1.2 | 1 | 3.7 | 0 | 0 | |||
| Prostate | 1 | 1.2 | 1 | 3.7 | 0 | 0 | |||
| Sarcoma | 1 | 1.2 | 0 | 0 | 1 | 1.8 | |||
| Cardiac arrest history | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Cardiac arrhythmia | 10 | 12.1 | 3 | 11.1 | 7 | 12.5 | |||
| Chronic kidney disease | 7 | 8.4 | 0 | 0 | 7 | 12.5 | |||
| History of renal transplant and/or on dialysis | 3 | 3.6 | 0 | 0 | 3 | 5.4 | |||
| Chronic pain (other than fibromyalgia) | 2 | 2.4 | 0 | 0 | 2 | 3.6 | |||
| Cirrhosis | 1 | 1.2 | 1 | 3.7 | 0 | 0 | |||
| Congestive heart failure | 4 | 4.8 | 0 | 0 | 4 | 7.1 | |||
| COPD | 2 | 2.4 | 0 | 0 | 2 | 3.6 | |||
| CAD | 9 | 10.8 | 1 | 3.7 | 8 | 14.3 | |||
| Dementia | 6 | 7.2 | 0 | 0 | 6 | 10.7 | |||
| Depression | 12 | 14.5 | 2 | 7.4 | 10 | 17.9 | |||
| Diabetes (gestational) | 2 | 2.4 | 1 | 3.7 | 1 | 1.8 | |||
| Diabetes (type I) | 4 | 4.8 | 0 | 0 | 4 | 7.1 | |||
| Diabetes (type II) | 17 | 20.5 | 4 | 14.8 | 13 | 23.2 | |||
| DVT history | 9 | 10.8 | 2 | 7.4 | 7 | 12.5 | |||
| Fibromyalgia | 3 | 3.6 | 1 | 3.7 | 2 | 3.6 | |||
| HCV | 1 | 1.2 | 1 | 3.7 | 0 | 0 | |||
| Hypertension | 34 | 40.1 | 7 | 25.9 | 27 | 48.2 | |||
| Intellectual disability | 1 | 1.2 | 0 | 0 | 1 | 1.8 | |||
| Myocardial infarction history | 1 | 1.2 | 0 | 0 | 1 | 1.8 | |||
| NAFLD | 1 | 1.2 | 1 | 3.7 | 0 | 0 | |||
| Peripheral vascular disease | 2 | 2.4 | 0 | 0 | 2 | 3.6 | |||
| Primary sclerosing cholangitis | 1 | 1.2 | 0 | 0 | 1 | 1.8 | |||
| Pulmonary embolism history | 6 | 7.2 | 1 | 3.7 | 5 | 8.9 | |||
| Schizophrenia | 5 | 6.0 | 1 | 3.7 | 4 | 7.1 | |||
| Stroke history | 4 | 4.8 | 1 | 3.7 | 3 | 5.4 | |||
| IBD medications taken in 2020 | 82 | 26 | 56 | ||||||
| No IBD meds taken in 2020 | 41 | 50.0 | 14 | 53.8 | 27 | 48.2 | |||
| 5-aminosalicylate | |||||||||
| Mesalamine | 24 | 29.3 | 7 | 26.9 | 17 | 30.4 | |||
| Sulfasalazine | 5 | 6.1 | 0 | 0 | 5 | 8.9 | |||
| Antibiotics (only for IBD) | |||||||||
| Ciprofloxacin | 2 | 2.4 | 0 | 0 | 2 | 3.6 | |||
| Metronidazole | 2 | 2.4 | 0 | 0 | 2 | 3.6 | |||
| Biologic therapy | |||||||||
| Adalimumab (Humira) | 5 | 6.1 | 2 | 7.7 | 3 | 5.4 | |||
| Certolizumab pegol (Cimzia) | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Golimumab (Simponi) | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Infliximab (Remicade) | 4 | 4.9 | 2 | 7.7 | 2 | 3.6 | |||
| Infliximab biosimilar (Renflexis, Inflectra) | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Natalizumab (Tysabri) | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Ustekinumab (Stelara) | 1 | 1.2 | 0 | 0 | 1 | 1.8 | |||
| Vedolizumab (Entyvio) | 3 | 3.7 | 1 | 3.8 | 2 | 3.6 | |||
| Corticosteroids (only for IBD) | |||||||||
| Budesonide (oral) | 2 | 2.4 | 1 | 3.8 | 1 | 1.8 | |||
| Prednisone or prednisolone (oral) | 12 | 14.6 | 2 | 7.7 | 10 | 17.9 | |||
| Rectal steroids | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Immunomodulator | |||||||||
| 6-MP | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Azathioprine | 1 | 1.2 | 0 | 0 | 1 | 1.8 | |||
| Cyclosporine | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Methotrexate | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Tacrolimus | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Tofacitinib (JAK inhibitor) | 1 | 1.2 | 0 | 0 | 1 | 1.8 | |||
aCalculated from first weight recorded in 2020. bExcluding non-melanoma skin cancer. cIncludes one colon cancer with brain metastases disease. AIDS: acquired immunodeficiency syndrome; BMI: body mass index; CAD: coronary artery disease; CD: Crohn’s disease; COVID-19: coronavirus disease 2019; DVT: deep vein thrombosis; ED: emergency department; HCV: hepatitis C virus; IBD: inflammatory bowel disease; JAK: Janus kinase; NAFLD: nonalcoholic fatty liver disease; SD: standard deviation; UC: ulcerative colitis; 6-MP: 6-mercaptopurine.
Among the hospitalized IBD patients, GI symptoms included diarrhea (39.3%), nausea (33.9%), and abdominal pain (25%). The mean length of hospitalization was 12.1 days, with 21.4% requiring ICU care, 35.7% experiencing AKI, and 16.1% requiring ventilator support. This can be compared with 19.5% of all COVID-19 inpatients system-wide requiring mechanical ventilation during this timeframe. Overall, 19.6% of the hospitalized IBD patients died, compared with 22.2% of all hospitalized COVID-19 patients. Details of the 11 IBD patients who died can be found in Supplementary Material 1 (www.gastrores.org). During their hospitalization, many IBD patients received azithromycin (32.1%) and most received hydroxychloroquine (67.9%), which has not been shown to influence the natural history of COVID-19 infection. There was no association between preadmission corticosteroid use or biologic treatment with a severe course of COVID-19 (Table 2). No patients who had received corticosteroids for their IBD died, stayed in the ICU, or were ventilated. Male sex (P = 0.03), age > 65 years (P = 0.01), and smoking (P = 0.02) were associated with AKI. Obesity (P = 0.03) was inversely associated with the composite adverse COVID-19 outcomes variable. Immunomodulator and tofacitinib exposure was identified in only two patients, limiting statistical analysis.
Table 2. Association Between Potential Risk Factors and Adverse Outcomes During COVID-19 Hospitalization.
| COVID-19 outcome | Risk factor | OR | 95% CI | P value |
|---|---|---|---|---|
| Death | Demographics | |||
| Age > 65 years | 2.88 | 0.73 - 11.32 | 0.18 | |
| Male sex | 2.00 | 0.51 - 7.80 | 0.31 | |
| Non-white race | 0.49 | 0.05 - 4.54 | 1.00 | |
| Health characteristics | ||||
| Obese | 0.12 | 0.01 - 1.00 | 0.04 | |
| Current or prior smoker | 2.40 | 0.46 - 12.58 | 0.36 | |
| UC IBD diagnosis | 0.65 | 0.17 - 2.55 | 0.74 | |
| Comorbidities | ||||
| Anxiety | 0.35 | 0.04 - 3.07 | 0.43 | |
| Depression | 1.03 | 0.19 - 5.69 | 1.00 | |
| Diabetes | 0.40 | 0.08 - 2.10 | 0.47 | |
| DVT and/or PE history | 3.10 | 0.71 - 13.48 | 0.20 | |
| Hypertension | 0.87 | 0.23 - 3.27 | 0.84 | |
| IBD medications | ||||
| No IBD medications | 2.19 | 0.56 - 8.54 | 0.25 | |
| Biologic therapy | 1.78 | 0.30 - 10.67 | 0.61 | |
| Anti-TNF biologic therapya | 1.40 | 0.13 - 14.92 | 1.00 | |
| 5-aminosalicylates | 0.28 | 0.05 - 1.43 | 0.17 | |
| Corticosteroids | b | b | 0.10 | |
| ICU stay | Demographics | |||
| Age > 65 years | 1.44 | 0.40 - 5.20 | 0.57 | |
| Male sex | 2.40 | 0.63 - 9.16 | 0.19 | |
| Non-white race | c | c | 0.17 | |
| Health characteristics | ||||
| Obese | 0.34 | 0.06 - 1.80 | 0.28 | |
| Current or prior smoker | 0.91 | 0.16 - 5.20 | 1.00 | |
| UC IBD diagnosis | 0.55 | 0.14 - 2.09 | 0.37 | |
| Comorbidities | ||||
| Anxiety | 1.50 | 0.33 - 6.82 | 0.69 | |
| Depression | 1.76 | 0.38 - 8.19 | 0.43 | |
| Diabetes | 0.64 | 0.15 - 2.74 | 0.73 | |
| DVT and/or PE history | 0.31 | 0.04 - 2.69 | 0.42 | |
| Hypertension | 1.10 | 0.31 - 3.93 | 0.89 | |
| IBD medications | ||||
| No IBD medications | 1.68 | 0.46 - 6.12 | 0.43 | |
| Biologic therapy | 0.58 | 0.06 - 5.31 | 1.00 | |
| Anti-TNF biologic therapya | 1.24 | 0.12 - 13.15 | 1.00 | |
| 5-aminosalicylates | 0.44 | 0.10 - 1.84 | 0.33 | |
| Corticosteroids | b | b | 0.10 | |
| Mechanical ventilation | Demographics | |||
| Age > 65 years | 1.08 | 0.26 - 4.54 | 1.00 | |
| Male sex | 2.27 | 0.51 - 10.18 | 0.47 | |
| Non-white race | c | c | 0.32 | |
| Health characteristics | ||||
| Obese | 0.61 | 0.11 - 3.52 | 0.69 | |
| Current or prior smoker | 0.52 | 0.05 - 4.89 | 1.00 | |
| UC IBD diagnosis | 0.57 | 0.13 - 2.55 | 0.72 | |
| Comorbidities | ||||
| Anxiety | 1.21 | 0.21 - 6.81 | 1.00 | |
| Depression | 1.39 | 0.24 - 7.98 | 0.66 | |
| Diabetes | 0.55 | 0.10 - 2.98 | 0.70 | |
| DVT and/or PE history | 0.46 | 0.05 - 4.15 | 0.67 | |
| Hypertension | 0.83 | 0.20 - 3.50 | 1.00 | |
| IBD medications | ||||
| No IBD medications | 0.83 | 0.20 - 3.50 | 1.00 | |
| Biologic therapy | 0.85 | 0.09 - 8.09 | 1.00 | |
| Anti-TNF biologic therapya | 1.83 | 0.17 - 19.91 | 0.51 | |
| 5-aminosalicylates | 0.74 | 0.16 - 3.31 | 1.00 | |
| Corticosteroids | b | b | 0.18 | |
| Composite adverse outcomes variablee | Demographics | |||
| Age > 65 years | 2.14 | 0.66 - 6.95 | 0.20 | |
| Male sex | 2.04 | 0.62 - 6.69 | 0.24 | |
| Non-white race | 0.24 | 0.03 - 2.12 | 0.25 | |
| Health characteristics | ||||
| Obese | 0.19 | 0.04 - 0.95 | 0.03 | |
| Current or prior smoker | 1.84 | 0.44 - 7.76 | 0.45 | |
| UC IBD diagnosis | 0.66 | 0.20 - 2.17 | 0.50 | |
| Comorbidities | ||||
| Anxiety | 0.92 | 0.21 - 4.04 | 1.00 | |
| Depression | 1.89 | 0.45 - 7.86 | 0.45 | |
| Diabetes | 0.38 | 0.09 - 1.57 | 0.17 | |
| DVT and/or PE history | 1.57 | 0.39 - 6.34 | 0.71 | |
| Hypertension | 0.54 | 0.17 - 1.78 | 0.31 | |
| IBD medications | ||||
| No IBD medications | 2.25 | 0.69 - 7.42 | 0.18 | |
| Biologic therapy | 1.00 | 0.17 - 5.77 | 1.00 | |
| Anti-TNF biologic therapya | 0.82 | 0.08 - 8.55 | 1.00 | |
| 5-aminosalicylates | 0.41 | 0.11 - 1.48 | 0.17 | |
| Corticosteroids | b | b | 0.02 |
aAdalimumab and infliximab. bUnable to be calculated due to 0 cell count. No patients who had received corticosteroids for IBD died, stayed in the ICU, or were ventilated. cUnable to be calculated due to 0 cell count. No non-white patients stayed in the ICU or were ventilated. dDefined as an increase in serum creatinine by ≥ 0.3 mg/dL within 48 h or an increase in serum creatinine to ≥ 1.5 times baseline within the prior 7 days compared with the preceding 1 year of data in acute care medical records. Does not include patients with a pre-existing diagnosis of end-stage kidney disease. eComposite adverse outcomes variable included at least one of the following: death, ICU stay, mechanical ventilator use. CI: confidence interval; DVT: deep vein thrombosis; IBD: inflammatory bowel disease; COVID-19: coronavirus disease 2019; ICU: intensive care unit; OR: odds ratio; PE: pulmonary embolism; TNF: tumor necrosis factor; UC: ulcerative colitis.
Discussion
In this cohort of IBD patients hospitalized with confirmed COVID-19 from a New York City metropolitan area health care system, we did not observe any association between negative outcomes and pre-admission IBD medication use. The mortality rate for IBD patients was similar to the overall patient population treated within the health care system over the same time period. Notably we observed a relatively low proportion of biologic and immunosuppressive medication use by our exclusively inpatient cohort (12.5% and 3.6%, respectively). This is in contrast to other regional data. Lukin et al reporting on 80 confirmed or suspected IBD/COVID-19 patients, including 17 inpatients, described rates of immunosuppressant use of 27.5% [29]. Axelrad et al’s report of 83 IBD/COVID-19 patients (54% confirmed, 46% suspected), including five hospitalized patients, noted that 70% were on a biologic agent, including 53% on an anti-tumor necrosis factor (TNF) agent [20]. The low proportion of immunosuppressant use observed in hospitalized patients with IBD/COVID-19 suggests that these medications may be protective against the development of more severe COVID-19. Additionally, the prior New York City reports were from single hospitals, each of which is home to a large dedicated IBD referral center, which would likely represent a more concentrated population of complex IBD patients on immune therapies. More recently, Ungaro et al have updated the earlier SECURE-IBD registry findings. Among over 1,400 patients, the authors found that combination therapy and thiopurines may be associated with an increased risk of severe COVID-19. On multivariable analysis, TNF antagonist therapy was not significantly associated with severe COVID-19, and there were no significant differences between biological classes (TNF, interleukin-12/23 and integrin antagonists) on the risk of severe COVID-19 [30].
Most of the study limitations are those common with retrospective analysis of IBD patients and outcomes. Specifically, the absence of any validated disease activity measures such as the Harvey Bradshaw Index or the Mayo score for CD and UC, respectively. As such we cannot determine how IBD activity may have impacted patient outcomes. However, even if such scoring was available, given the high frequency of GI complaints that have been observed within COVID-19 populations, it is unlikely that these activity measures could be regarded as valid in this setting. Similarly, we were unable to provide disease assessment by sigmoidoscopy or colonoscopy. None of our cohort had a lower GI endoscopic examination during their admission, as was standard practice for all but the most critical conditions during the peak of the pandemic.
Another limitation lies in the statistical methods able to be employed in this study. Due to our limited sample size and the exploratory nature of this work, the goal of our analysis was to be descriptive and to identify associations between patient characteristics and outcomes, thus Chi-square and Fisher’s exact tests were employed rather than predictive models. While this initial work has identified some IBD patient factors that are associated with and may be predictive of the outcome, future studies with larger sample sizes and the utilization of logistic regression models would be needed to build upon the current data to draw reliable predictive outcomes.
Against the study limitations are several key strengths. As noted, the large population receiving care for COVID-19 within the health care system likely provides a valid representation of the inpatient COVID-19 experience in the region generally, and the IBD/COVID-19 population specifically. However, the numbers of IBD/COVID-19 patients themselves were at a scale small enough to allow direct chart review of all cases, to validate both the IBD and COVID-19 diagnoses. The large number of cases identified by ICD-10 code search and then excluded after direct chart review (44.7%) illustrates the hazards of relying upon automated data extraction alone. Similarly, we used a highly stringent standard for COVID-19 diagnosis, including only those with confirmed positive PCR testing. While this may have excluded some true IBD/COVID-19 patients, this is balanced against the greater certainty of an accurate diagnosis in those remaining. Also, the universal testing of all patients coming to the hospital during the pandemic, and the inclusion of all identified hospitalized IBD/COVID-19 patients in our analysis, excludes the possibility of reporting bias.
Conclusion
In conclusion, our analysis of a large inpatient cohort of IBD patients admitted with COVID-19 did not find any association between pre-admission use of corticosteroids, biologics, or immunosuppressants and adverse outcomes. While the mortality rate was high for IBD/COVID-19 patients, it was not greater than for hospitalized COVID-19 patients as a whole. Though our results are encouraging, we continue to support the recommendations of the leading GI and IBD societies to regard our patients as “at risk”, and to observe caution in their care.
Supplementary Material
Description of IBD Patients Who Died During a COVID-19 Hospitalization (n = 11).
Acknowledgments
None to declare.
Financial Disclosure
A. Swaminath received advanced IBD fellowship support from Janssen and Takeda.
Conflict of Interest
None to declare.
Informed Consent
For this type of study formal consent is not required.
Author Contributions
KS conceived of the idea for the study and drafted the first version of the manuscript. KS, AS, JA, LD, AM, and BK designed the study. KS, AS, JA, AT, and LD supervised the data collection and coordinated the collaboration between the participating institutions. RB, JM, NB, MA, KL, and LD conducted chart reviews and collected the data. LD performed the statistical analyses. All authors critically reviewed and edited the final version of the manuscript. All authors read and approved the final version of the manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
- IBD
inflammatory bowel disease
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- 2019-nCoV
2019 novel coronavirus
- COVID-19
coronavirus disease 2019
- ACE2
angiotensin-converting enzyme 2
- GI
gastrointestinal
- SECURE-IBD
Surveillance Epidemiology of Coronavirus Under Research Exclusion for IBD
- CD
Crohn’s disease
- UC
ulcerative colitis
- PCR
polymerase chain reaction
- ICU
intensive care unit
- AKI
acute kidney injury
References
- 1. Coronavirus disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/index.html. Accessed October 6, 2020.
- 2. WHO Director-General's opening remarks at the media briefing on COVID-19. March 11, 2020.
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morens DM, Daszak P, Taubenberger JK. Escaping Pandora's box - another novel coronavirus. N Engl J Med. 2020;382(14):1293–1295. doi: 10.1056/NEJMp2002106. [DOI] [PubMed] [Google Scholar]
- 7.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7):e00127-20. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang W, Feng Z, Rao S, Xiao C, Xue X, Lin Z, Zhang Q. et al. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69(6):1141–1143. doi: 10.1136/gutjnl-2020-320832. [DOI] [PubMed] [Google Scholar]
- 9.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833.e1. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suarez-Farinas M, Tokuyama M, Wei G, Huang R, Livanos A, Jha D, Levescot A. et al. Intestinal inflammation modulates the expression of ACE2 and TMPRSS2 and potentially overlaps with the pathogenesis of SARS-CoV-2-related disease. Gastroenterology. 2021;160(1):287–301.e220. doi: 10.1053/j.gastro.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahiya DS, Kichloo A, Albosta M, Pagad S, Wani F. Gastrointestinal implications in COVID-19. J Investig Med. 2020;68(8):1397–1401. doi: 10.1136/jim-2020-001559. [DOI] [PubMed] [Google Scholar]
- 12.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 13.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D. et al. Baseline Characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy NA, Jones GR, Lamb CA, Appleby R, Arnott I, Beattie RM, Bloom S. et al. British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut. 2020;69(6):984–990. doi: 10.1136/gutjnl-2020-321244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin DT, Feuerstein JD, Wang AY, Cohen RD. AGA clinical practice update on management of inflammatory bowel disease during the COVID-19 pandemic: expert commentary. Gastroenterology. 2020;159(1):350–357. doi: 10.1053/j.gastro.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin DT, Abreu MT, Rai V, Siegel CA, International Organization for the Study of Inflammatory Bowel D. Management of patients with Crohn's disease and ulcerative colitis during the coronavirus disease-2019 pandemic: results of an international meeting. Gastroenterology. 2020;159(1):6–13.e16. doi: 10.1053/j.gastro.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allocca M, Fiorino G, Zallot C, Furfaro F, Gilardi D, Radice S, Danese S. et al. Incidence and patterns of COVID-19 among inflammatory bowel disease patients from the Nancy and Milan cohorts. Clin Gastroenterol Hepatol. 2020;18(9):2134–2135. doi: 10.1016/j.cgh.2020.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taxonera C, Sagastagoitia I, Alba C, Manas N, Olivares D, Rey E. 2019 novel coronavirus disease (COVID-19) in patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2020;52(2):276–283. doi: 10.1111/apt.15804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marafini I, Salvatori S, Sena G, Calabrese E, Biancone L, Monteleone G. Low frequency of COVID-19 in inflammatory bowel diseases. Dig Liver Dis. 2020;52(11):1234–1235. doi: 10.1016/j.dld.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Axelrad JE, Malter L, Hong S, Chang S, Bosworth B, Hudesman D. From the American epicenter: coronavirus disease 2019 in patients with inflammatory bowel disease in the New York City metropolitan area. Inflamm Bowel Dis. 2021;27(5):662–666. doi: 10.1093/ibd/izaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mak JWY, Weng MT, Wei SC, Ng SC. Zero COVID-19 infection in inflammatory bowel disease patients: Findings from population-based inflammatory bowel disease registries in Hong Kong and Taiwan. J Gastroenterol Hepatol. 2021;36(1):171–173. doi: 10.1111/jgh.15164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guerra I, Algaba A, Jimenez L, Mar Aller M, Garza D, Bonillo D, Molina Esteban LM. et al. Incidence, clinical characteristics, and evolution of SARS-CoV-2 infection in patients with inflammatory bowel disease: a single-center study in Madrid, Spain. Inflamm Bowel Dis. 2021;27(1):25–33. doi: 10.1093/ibd/izaa221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norsa L, Cosimo P, Indriolo A, Sansotta N, D'Antiga L, Callegaro A. Asymptomatic severe acute respiratory syndrome coronavirus 2 infection in patients with inflammatory bowel disease under biologic treatment. Gastroenterology. 2020;159(6):2229–2231.e2222. doi: 10.1053/j.gastro.2020.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aziz M, Fatima R, Haghbin H, Lee-Smith W, Nawras A. The incidence and outcomes of COVID-19 in IBD patients: a rapid review and meta-analysis. Inflamm Bowel Dis. 2020;26(10):e132–e133. doi: 10.1093/ibd/izaa170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, Ng SC. et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159(2):481–491.e483. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bezzio C, Saibeni S, Variola A, Allocca M, Massari A, Gerardi V, Casini V. et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020;69(7):1213–1217. doi: 10.1136/gutjnl-2020-321411. [DOI] [PubMed] [Google Scholar]
- 27.Singh S, Khan A, Chowdhry M, Bilal M, Kochhar GS, Clarke K. Risk of severe coronavirus disease 2019 in patients with inflammatory bowel disease in the United States: a multicenter research network study. Gastroenterology. 2020;159(4):1575–1578.e1574. doi: 10.1053/j.gastro.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, the Northwell C-RC. et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lukin DJ, Kumar A, Hajifathalian K, Sharaiha RZ, Scherl EJ, Longman RS, Jill Roberts Center Study Group Study G. et al. Baseline Disease activity and steroid therapy stratify risk of COVID-19 in patients with inflammatory bowel disease. Gastroenterology. 2020;159(4):1541–1544.e1542. doi: 10.1053/j.gastro.2020.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ungaro RC, Brenner EJ, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, Ng SC. et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut. 2021;70(4):725–732. doi: 10.1136/gutjnl-2020-322539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of IBD Patients Who Died During a COVID-19 Hospitalization (n = 11).
Data Availability Statement
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.