Abstract
Background:
Chronic methicillin resistant Staphylococcus aureus (MRSA) in CF is associated with worse outcomes compared to early or intermittent infection. This observation could be related to adaptive bacterial changes such as biofilm formation or anaerobic growth.
Methods:
MRSA isolates stored from incident and during chronic (>2 years) infection were included at two study sites. MRSA isolates were characterised by spa-typing, antimicrobial susceptibility testing, biofilm formation and haemolysis under aerobic and anaerobic culture conditions.
Results:
Paired MRSA isolates from 49 patients were included. Mean age at incident infection was 9.7±1.2 years with mild to moderate lung disease (FEV1 74±4% predicted). Twenty-five subjects showed progression of disease/symptoms after onset of MRSA with significantly increased use of antibiotics. Most isolates belonged to t002 (38%) and t008 (36%) spa-types and 8 patients had a change in spa-type over time. Antimicrobial susceptibility testing showed few differences between incident and late isolates but significantly lower MIC under anaerobic vs. aerobic conditions for vancomycin, fusidic acid, rifampin but higher MIC for trimethoprim-sulfamethoxazole. Biofilm formation and haemolysis did not differ by stage of infection or disease course but both were lower under anaerobic conditions (biofilm p=0.018; haemolysis p=0.002) in multi-variate analyses that included study site, growth condition and stage of infection.
Conclusions:
Persistent MRSA infection is frequently associated with clinical decline. Anaerobic growth conditions, which occur in CF airways, affect the expression of virulence factors and antibiotic susceptibility of MRSA more than duration of infection.
Keywords: Staphylococcus aureus, Antimicrobial resistance, Anaerobe, Haemolysis, Adaptation, Outcome
INTRODUCTION
Staphylococcus aureus (SA) is often the earliest bacterium detected in respiratory cultures of people with cystic fibrosis (CF). In the U.S., the rate of methicillin-resistant SA (MRSA) detection is around 25%. Whereas intermittent respiratory infection with MRSA does not appear to affect patient outcomes significantly, chronic MRSA infection (i.e., MRSA positive cultures for ≥ 2 years) is associated with higher mortality rates than for patients without MRSA or those with intermittent MRSA infection1. Cross-sectional studies have identified significant associations between MRSA and worse pulmonary outcomes, including lower lung function and the failure to recover to baseline at times of exacerbation in subjects with MRSA compared to those with methicillin susceptible SA (MSSA)2, 3. Further, our earlier observational study showed that clinical outcomes were unchanged during the initial 6 months after initial MRSA infection, but started worsening around 2 years thereafter, and that the magnitude of this effect differed by molecular type of MRSA4. These observations suggest that prolonged infection is a determinant of worse clinical outcomes, which may be due to adaptation of MRSA in the CF airways.
The CF lung environment is characterized by mucus obstruction and intense neutrophilic inflammation, resulting in anaerobic niches that support the growth of facultative anaerobic and strictly anaerobic bacteria5–8. People with CF receive frequent antibiotics that enhance pressure on the bacteria for survival. S. aureus is a versatile organism, exhibiting multiple adaptive growth mechanisms and phenotypes, including changes in metabolism and antibiotic susceptibilities, and enhanced capacities for biofilm formation and anaerobic growth9, 10. While the growth characteristics cited above have been identified among isolates from CF lung infections11–13, the relationships between MRSA phenotypes, chronic infection and CF clinical outcomes are less clearly defined.
Here we hypothesized that phenotypic changes of MRSA occur during the transition from incident (< 6 months) to chronic (>2 positive cultures over >1 years) infection and that these are associated with changes in patient outcomes. Further, we tested whether anaerobic growth of these isolates, as is believed to occur in CF lung infections, affect phenotypes associated with virulence and antibiotic susceptibility. To this end, we compared key growth characteristics using bacterial isolates cultured at time of incident MRSA detection and those collected 2–3 years later from the same patients.
METHODS
Subjects and clinical data
Stored MRSA isolates available at the respective microbiology laboratories were included from subjects attending the CF centers at the University of NC, Chapel Hill (UNC) and at the University of Washington, Seattle (UW). Isolates were from subjects who had had an incident (first life-time or at least 2 years negative prior to this time-point) isolation of MRSA between 2006 and 2014 and remained MRSA-infected at least 2 years later. Subjects who had undergone organ transplant were excluded. Institutional ethics review boards at both institutions considered the study exempt from obtaining consent.
Patients’ clinical information was abstracted from their medical records for anthropometrics, lung function (specifically, forced expiratory volume in 1 second, FEV1% predicted)14, antibiotics given during the study period, and co-infection with other bacteria for at least a 6 month time period around incident and chronic isolate collection; in many patients data from additional visits within the study period were also available.
Subjects were classified as those with Stable (S) vs. Progressive (P) disease after initial MRSA isolation based on clinical outcomes in the 2 years after incident MRSA. Subjects categorized as progressive disease were those who were prescribed antibiotics for new changes in respiratory symptoms at >50% of clinic visits, and/or had a decrease in body mass index (BMI) percentile between the collection of incident and chronic isolates. For subjects >6 years with spirometry results reported, those with a ≥5% relative decline in FEV1 % predicted during study period were also categorized as progressive disease.
Bacterial isolates:
Incident isolates of MRSA were stored per protocol of the respective clinical microbiology laboratories. At UNC, MRSA isolates were stored at incident infection and at yearly intervals if the patient remained infected. At UW, MRSA isolates were collected during routine quarterly care visits of local CF patients and archived as is routinely performed by the Center for CF Microbiology at Seattle Children’s Hospital, with associated clinical data maintained in the UW CF center clinical database. Stored isolates that were reported by the clinical laboratory to be small-colony variants (SCV) were not included given this study’s focus on phenotypes that would be impacted by the altered growth rates, protein expression, and metabolic activities of SCVs.
In vitro assays
Confirmation of MRSA
Isolates were confirmed to be MRSA by determination of oxacillin resistance by E-Test (≥8μg/ml) following PCR detection of the mecA gene15. Presence or absence of Panton-Valentin Leukocidin toxin was determined by PCR detection of lukS-V using previously published primers16. Bacteria were cultured on Mueller-Hinton agar either aerobically or in an anaerobic workstation (Don Whitley A35 using 10% H2, 10% CO2, and 80 % N2) at 37°C for antibiotic susceptibility testing, biofilm formation and haemolysis.
Spa typing
Following DNA extraction, spa types of all isolates were determined as previously described17, and assigned using the Ridom-Staph software (http://spaserver.ridom.de). Using the integrated BURP (Based Upon Repeat Patterns) algorithm, spa types were clustered into clonal complexes (spa-CC). Spa types were assigned to the same cluster if cost distances were less than four, and repeats of shorter than five were excluded from the analysis18.
Biofilm and haemolysis assays
These phenotypic assays were carried out following culture in both aerobic and anaerobic growth conditions for each isolate, in each case in triplicate in Mueller Hinton Broth. Biofilm formation of each isolate was determined by crystal violet staining of adherent cells after 24hrs in a 96 well plate format, as described previously19. δ-haemolysin activity was measured as a surrogate marker for agr function as in previous work20. Specifically, bacteria were grown to log phase either aerobically or anaerobically, and brought to an OD600nm of 0.15, equivalent to 1×108 cfu/ml. Following centrifugation at 2000g for 5 min, supernatant was removed and heated at 100°C for 5 minutes in order to inactivate heat-labile haemolysins (e.g. α-toxin). The supernatant was then added to a solution of 4% v/v sheep red blood cells and incubated at 37°C for 1 hour. The percentage of sample haemolysis was calculated as the sample (OD580nm/positive control OD580nm) x 100%, where the positive control was generated by addition of Triton-X to the red blood cells.
Antibiotic susceptibility testing
Minimal inhibitory concentrations (MICs) of antibiotics commonly used in clinical practice were determined by E-test™ in accordance with the manufacturer’s instructions and were carried out under both aerobic and anaerobic conditions for vancomyin, linezolid, trimethoprim-sulfamethoxazole (TMP-SMX), fusidic acid, rifampin, and clindamycin. We used the criteria of the Clinical and Laboratory Institute on Antimicrobial Susceptibility Testing (CLSI) for classification as susceptible vs. resistant except for fusidic acid for which only criteria of the European Committee on Antimicrobial Susceptibility Testing are available.
Statistical analyses:
Samples were described as mean±standard error of the mean (SEM), unless otherwise stated. Characteristics at baseline between stable and progressive disease were compared with Fisher’s exact test, 2 sample t-tests or non-parametric equivalent (Mann-Whitney and Wilcoxon t-test). Comparisons between sites were done with 2 samples t-tests. Comparison of bacterial phenotypes and MIC between incident and chronic samples were done with paired tests (t-test or Wilcoxon test as appropriate for the variable). Multi-variate analyses that included study site, growth condition and stage of infection were included in a repeated measure model.
All tests were 2-sided and p-values < 0.05 were considered significant. Statistics were performed using Excel V2016 and JMP Pro12 with graphics done in GraphPad Prism V8.
RESULTS
1. Subjects
Paired isolates from 49 subjects (30 UNC, 19 UW) were included of whom two were adults at incident sample. The source of culture for the 98 isolates was bronchoalveolar lavage for 5, oropharyngeal swabs for 52 and sputum for 41 samples. Mean (±SEM) age at time of incident MRSA detection was 9.7±1.2 years, and source subjects presented with mild to moderate disease based on lung function (74±4%) in the 34 patients old enough to perform spirometry at the early timepoint. Twenty-four subjects showed no relevant clinical change over the duration of the follow-up and were categorized as S, whereas 25 did show a progression in terms of spirometry, need for antibiotics, or BMI, and were classified as P. The proportion of subjects homozygous for the F508del CFTR mutation was 58%; 48% were heterozygotes with 8% of subjects F508del negative in each group. Genotype distribution did not differ between those with progressive (46% homo- and 46% heterozygote) and stable disease (61% homo- and 30% heterozygote) p=0.06. As expected from the definition of the progressive disease, these subjects received significantly more antibiotics in the interval from incident to chronic sample (5.13±0.43 vs. 2.04±0.42, p<0.0001), and a higher proportion of antibiotic prescriptions per clinic visit (0.75±0.05 vs. 0.38±0.05, p<0.0001). A variety of antibiotics was prescribed, with TMP-SMX being used in over a third of the visits (39% at UNC and 35% at UW).
Thirty-one subjects had matched incident-chronic time points for lung function (Figure S1). Those with progressive disease (n=16) had a mean 0.04% relative decrease in FEV1% predicted, compared with a mean increase of 0.004% among those with stable disease (n=15) (p=0.56 for P vs. S).
Groupwise clinical characteristics are presented in Table 1.
Table 1.
Data presented as mean±SEM. Lung function as forced expiratory volume in 1st second (FEV1) expressed as % predicted to reference group14. Group-wise comparisons between progressive and stable groups were done at each time point with p-values given in the column of Stable.
| Characteristic | Incident time point+ | Chronic time point+ | ||
|---|---|---|---|---|
|
| ||||
| Progressive (n=24) | Stable (n=25) | Progressive (n=24) | Stable (n=25) | |
| Age (years) | 12.23±1.63 | 7.33±1.59 P=0.04 |
15.29±1.61 | 10.24±1.58 P=0.029 |
| Time interval Incident-Chronic+ | 3.07±0.39 | 2.91± 0.38 P=0.77 |
||
| FEV1 % predicted | 74.2±5.99 (n=18) | 83.0±6.4 (n=16) P=0.32 |
65.0±5.1 (n=19) | 86.2±5.2 (n=18) P=0.006 |
| BMI% ile | 38.39±6.03 | 45.9±5.91 P=0.37 |
36.94±5.10 | 54.55±5.00 P=0.017 |
| Chronic P. aeruginosa† | 3 (13%) | 7 (28%) P=0.29* |
5 (21%) | 9 (36%) P=0.35* |
“Incident” signifies initial detection of MRSA; “chronic” indicates a culture after the indicated interval.
Chronic P. aeruginosa was defined as ≥50% of cultures being P. aeruginosa positive in the last year21.
indicates results by Fisher’s exact test.
Subjects at the two study sites had similar age at incident MRSA (UNC 9.5±1.5 years and UW 10.0±1.9 years, p=0.85), similar BMI% iles (36.7±5.5 at UNC vs. 50.9±6.3 at UW, p=0.1) but those at UNC had lower FEV1% predicted compared to UW (66.9 ±4.9% n=19 vs. 94.1±5.5%, n=15; p<0.001). At collection of chronic isolates, differences in FEV1% predicted were less pronounced, yet significant: UNC 67.9±5.2%, n=20 vs. UW 83.9±5.6%, n=17, p=0.04. The ratio of clinic visits at which antibiotics were prescribed (0.52±0.05 at UNC vs. 0.61±0.07 at UW, p=0.31) and the proportion of subjects who showed progressive disease (16/30 vs. 8/19, p=0.6) were not different between UNC and UW.
2. MRSA genotypes
Ninety-eight isolates were included. At incident infection, spa typing categorized the majority of isolates into the t002 (38% and 29% of isolates at UNC and UW, respectively) and t008 (35% and 34% of isolates at UNC and UW, respectively, p=0.68) with a variety of other spa types for the remaining isolates. Spa type t008 includes clonal complex (CC) 8, which in the U.S. is most frequently USA30022. Figure 1 provides an overview for all isolates. Across all isolates, 22/49 (45%) isolates belonged to spa-CC8 and 23/49 (47%) belonged to spa-CC5 with 4 isolates having no founder or being singletons. Distributions of spa-clade were similar across sites (CC5: 45% UNC, 37% UW, CC8: 50% UNC, 50% UW; p=0.67). Over time, eight patients (16%) had a change in spa-CC, with 4 changing from spa-CC5 to spa-CC8 and 4 changing between singletons and spa-CC8. Sixty-two percent of isolates were pvl positive as measured by PCR.
Figure 1:
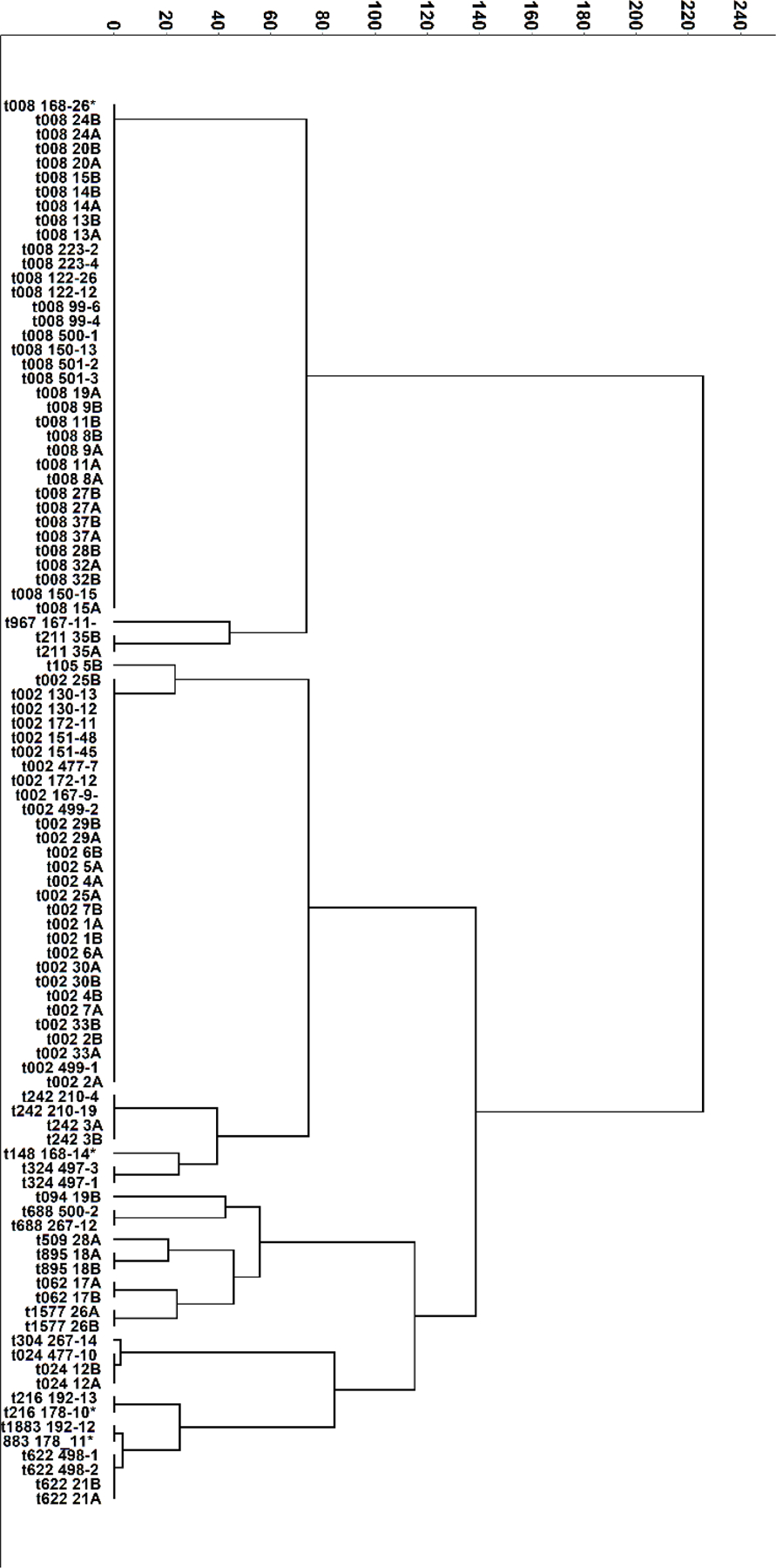
A dendrogram displaying the characteristics of the study isolates, including spa-type (text). Ridom-Staph software identified 18 unique spa-types for the 98 isolates. The dendrogram shown was constructed based on the degree of similarity between isolates, calculated using Hierarchical Cluster Analysis with Ward’s Method. The numbers next to spa-type indicate the patient identifier with those ending in A (incident) B (chronic) being from UNC and those with only numerical identifier from UW.
3. Antimicrobial susceptibility
Using CLSI breakpoints, no isolates were resistant to either vancomycin, linezolid or TMP-SMX at either time point under either aerobic or anaerobic growth conditions. One isolate from the chronic time point was resistant to fusidic acid. Resistance to rifampin increased over time; specifically, 4 patients had resistant incident isolates, compared to 6; i.e., an additional two patients (one S, one P disease) had emergent resistance at the chronic timepoint. High-level resistance to clindamycin occurred in 10 patients of whom four reverted to susceptible at the chronic time point (all four P). Six patients (5/6 P) developed non-highlevel resistance at the chronic time. None of the changes in resistance was accompanied by changes in spa-type.
There were no increases in MIC between incident and chronic isolates for any of the antibiotics (Table S1) by pairwise comparisons. Similarly, pairwise comparisons stratified by stable vs. progressive disease showed no MIC changes over time (Table S2). Groupwise comparisons showed no differences between incident and chronic isolate (Table S1). Groupwise comparisons by disease course showed lower MICs for TMP-SMX but higher MIC for linezolid and fusidic acid in stable vs. progressive disease (Table S3). For the entire isolate set MIC values were lower under anaerobic vs. aerobic conditions for vancomycin, fusidic acid, and rifampin but were higher under anaerobic conditions for TMP-SMX (Table 2).
Table 2.
Minimal Inhibitory Concentration under aerobic and anaerobic conditions
| MIC μg/mL | Vanco-mycin | Linezolid | Fusidic Acid *,† (n=91) | Rifampin* (n=82) |
TMP-SMX | Clindamycin* (n=69) |
|---|---|---|---|---|---|---|
| Aerobic | 0.74±0.02 | 1.14±0.06 | 0.09±0.007 | 0.04±0.02 | 0.04±0.02 | 0.06±0.002 |
| Anaerobic | 0.58±0.02 | 1.10±0.07 | 0.04±0.006 | 0.03±0.014 | 0.08±0.02 | 0.09±0.006 |
| p-value | <0.0001 | 0.63 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
MIC in μg/mL reported as mean ± standard error of the mean of triplicate testing for incident and chronic isolates.
Pairs with one or both resistant isolates per CLSI breakpoints were excluded.
EUCAST criteria as CLSI breakpoints for fusidic acid are not available. Comparisons between aerobic and anaerobic conditions are by Wilcoxon matched pairs signed rank test.
4. Bacterial growth characteristics:
Although all isolates showed the capacity to form biofilms to various degrees and some showed increases over time, no significant differences were observed between incident and chronic isolates in biofilm production either in group-wise or paired analyses (Figure S2). Biofilm production was lower under anaerobic compared to aerobic conditions (0.97±0.06 vs. 1.19±0.09, p=0.02), but did not differ by stage of infection (early 1.14±0.13 vs. late 1.23±0.13, p=0.06) (Figure 2). The extent of δ-haemolysin production within each oxygen condition differed between individual isolates but not by stage of infection (incident 1.14±0.13 vs. chronic 1.23±0.13, p=0.06) or by clinical outcomes (Figure 2). However, haemolysis was higher under aerobic compared to anaerobic conditions for all isolates (p<0.001) and for incident isolates (p=0.0003). Genetic background i.e. spa-CC did not affect biofilm formation under aerobic (CC8 1.33±0.13 vs. CC5 1.10±0.14,p=0.19) nor anaerobic (CC8 0.99±0.09 vs. CC5 0.98±0.09,p=0.94). Haemolysis was similar between spa-CC under aerobic (CC8 0.14±0.02 vs. CC5 0.16±0.02, p=0.48) and anaerobic (CC8 0.10±0.02 vs. CC5 0.09±0.02, p=0.74) conditions.
Figure 2:
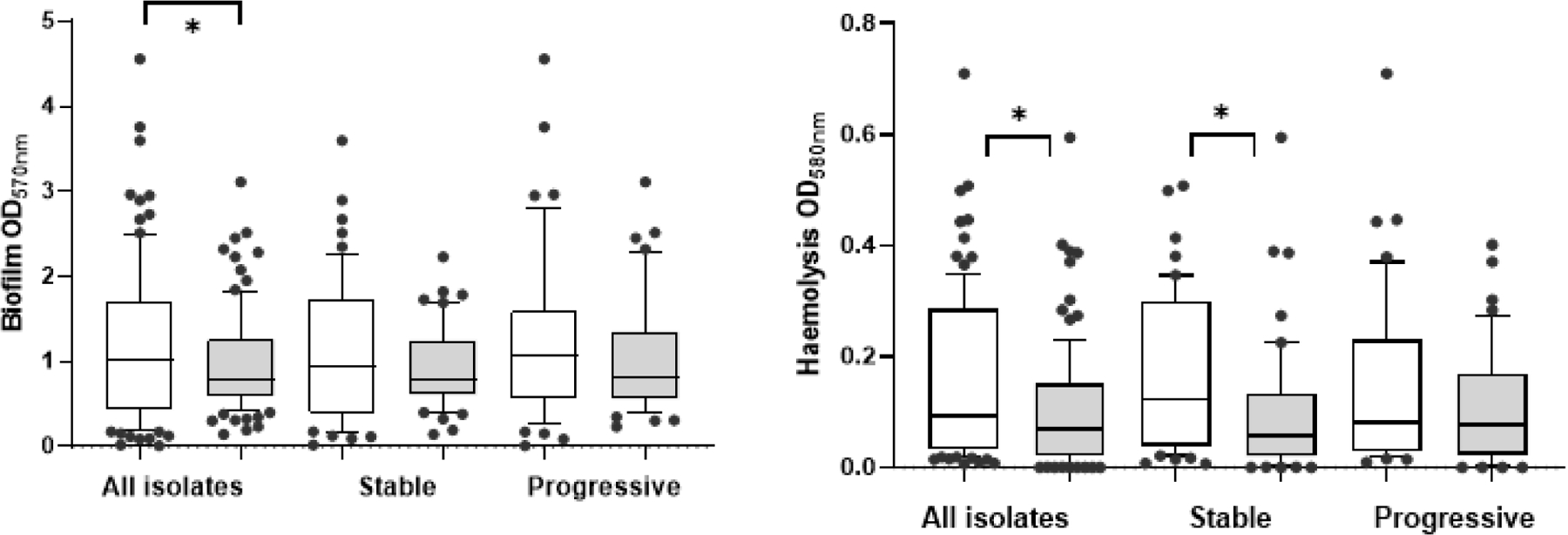
Biofilm (A) and Haemolysis (B) aerobic and anaerobic growth conditions. Comparisons by Wilcoxon test. Bars indicate mean ± SEM. Open bar indicates aerobic and shaded bar anaerobic conditions with scatter 10–90%.
Multi-variate analyses that included study site, growth condition and stage of infection showed that biofilm formation differed significantly by site (p=0.018) and aerobic vs anaerobic growth condition (p=0.0035), but haemolysis differed only in terms of aerobic vs. anaerobic growth (p=0.0017)(Table 3).
Table 3.
Multivariate model. Site is UW vs. UNC; O2 = aerobic, O2− = anaerobic growth conditions. CI = confidence interval.
| A: Biofilm | ||||
|---|---|---|---|---|
|
| ||||
| Effect | Estimate | SEM | 95% CI | p-value |
| Incident vs. Chronic | 0.095 | 0.126 | −0.154, 0.345 | 0.449 |
| Site | 0.386 | 0.129 | 0.131, 0.642 | 0.004 |
| O2 vs. O2− | −0.202 | 0.084 | −0.368, −0.035 | 0.018 |
| B: Haemolysis | ||||
|
| ||||
| Effect | Estimate | SEM | 95% CI | p-value |
|
| ||||
| Incident vs. Chronic | 0.008 | 0.020 | −0.033, 0.048 | 0.701 |
| Site | −0.005 | 0.021 | −0.047, 0.036 | 0.805 |
| O2 vs. O2− | −0.055 | 0.017 | −0.089, −0.021 | 0.002 |
DISCUSSION
In this study, we analysed MRSA isolates from subjects with CF collected at incident detection and at a late/chronic infection time point. We found that most subjects remained infected with the same spa type during the study, rather than switching MRSA strains. We identified little change in clinically relevant bacterial phenotypes over time (Fig. S1) or based on disease course (Fig. 2). Yet, haemolysis and antimicrobial susceptibilities were variably altered by oxygen availability.
Genetic and epidemiologic analysis showed the MRSA isolates in this study reflected MRSA strains occurring most commonly in the U.S.: t002 and t008 and as seen in our prior study23. Neither clinical outcomes nor phenotype differed by spa-clade. About 50% of the subjects had a progression of disease during transition from incident to chronic MRSA infection. The rate of P. aeruginosa infection was overall low in our mostly pediatric population without significant differences between groups (Table 1). However, the group with progressive disease was older at baseline, which may have affected their disease course. We did not find genotypic and phenotypic differences in MRSA between the groups, which may implicate host factors rather than specific MRSA phenotypes in disease progression.
Despite the high rate of antibiotic use (75% of visits among progressive disease), the antibiotic susceptibility profiles of the MRSA isolates did not change appreciably over time in terms of either resistances or group-wise MIC and we observed little change in the MICs of isolates from individual subjects over time (Tables S1, S2). However, we found growth conditions relevant for the CF airway altered antibiotic susceptibility more than stage of infection. Anaerobic growth affected antibiotic susceptibility differently according to antibiotic class. Under anaerobic conditions, isolates were more susceptible to vancomycin and fusidic acid but less susceptible to TMP-SMX. Similar drug-specific differences in susceptibility to individual drug components have been described for fosfomycin/tobramycin, where anaerobic conditions affected MIC and killing in opposite directions for each drug component. Decreased antimicrobial activity for TMP-SMX under anerobic compared to aerobic conditions has been documented before24. Therefore, while not specific to CF, anaerobic conditions likely contribute to the known poor correlation of in vitro antimicrobial susceptibilities to clinical outcomes in CF25.
Rifampin resistance increased slightly from incident to chronic infection, likely due to use of this antibiotic among the study subjects. Rifampin is generally used in combination with other drug classes26; therefore, this observation indicates that even as combination therapy, resistance to rifampin can emerge in MRSA. Interestingly, high-level resistance to clindamycin was less common among chronic compared to the incident isolates. This observation could be due to decreasing use of this antibiotic or macrolides that promote inducible resistance to clindamycin. Because growing evidence indicates a specific subtype of S. aureus known as small-colony variants are selected by TMP-SMX and associated with lower lung function compared to other S. aureus, we specifically excluded SCVs from our collection of isolates, and we observed no isolates in our collection exhibiting resistance to TMP-SMX. This may be explained by a comparatively lower use of TMP-SMX among the subjects we studied here than in studies that reported high prevalence of SCV SA11, 12.
Anaerobic conditions and the presence of obligate anaerobes in the airway secretions of people with CF have been well described6–8. As with many other microorganisms in CF, SA can adapt to anaerobic conditions, including by down-regulating metabolic activity. The decreased haemolytic activity observed here under anaerobic conditions may reflect such adaptation and/or decreased virulence. Two recent studies, each including longitudinal isolates from three patients, performed phenotypic analyses and confirmed changes in metabolism during adaptation to chronic infection27, 28. However, each of these studies identified increased biofilm formation over time in two of the three isolate series analyzed. We also noted inter-subject differences in biofilm formation but no differences by pairwise or groupwise comparisons of incident vs. chronic isolates from 49 subjects (Fig. S2). Of note, there are no standardized protocols for biofilm formation in SA making inter-study comparisons more difficult to interpret. Our observation that anaerobic growth alters δ-haemolytic activity is consistent with observations from previous studies, showing several virulence-associated genes to be regulated by oxygen availability. For example, the agr system in SA is a key regulator of several virulence factors, including δ-haemolysin, encoded by hld ; the expression of both genes has been shown to be modulated by oxygen29, 30. However, as with biofilm formation, we did not observe a difference in haemolytic activity with duration of infection. These observations are consistent with a prior study, which examined various phenotypic changes in SA (mostly methicillin sensitive, with a resultant higher breadth of spa types compared with our MRSA-focused study) from 29 people with CF during chronic infection13. For 7/29 subjects the incident isolate was available. Changes in biofilm formation and haemolysis occurred in isolates from fewer than half of those seven subjects- 41% and 45%, respectively- without reaching significance across all patients. As for most prior work on SA adaptation, this study performed all assays under aerobic conditions. Together with our work, the available evidence indicates a high degree of inter-subject variability in adaptive phenotypic change among SA isolates. Our results expand on this finding by showing that anaerobic growth affects these phenotypes more than duration of infection, indicating that spatial variation in the conditions within host airways may more strongly influence isolate behaviours that determine persistence, response to treatment, and clinical status.
Limitations of our study include our analysis, as per common clinical laboratory practice, of a single isolate at each time point31; as a result, we cannot exclude the possibility of genetic or phenotypic diversity that would have been apparent if we had used multiple isolates per sample. In support of our approach, multiple studies on population diversity of SA have shown among the majority of patients that one strain dominates within a sample32, 33. Our study was also limited by sample size, absence of lung function data from children <6 years, and the inclusion of two CF centers, which could limit generalizability. While our analysis showed some differences in results by site, for the majority of subjects the paired isolates were from the same spa-type of MRSA, supporting generalizability beyond these two centers.
We used spa typing as a rigorous, detailed typing method that provides results that correlates well with multi-locus sequencing typing. We noted some variations in spa coding sequence; i.e. genetic drift within patients’ isolates over time, similar to findings reported for MSSA isolates in CF34. Persistence of a dominant strain is also consistent with prior reports that used different staphylococcal typing methods33, 35, 36.
In conclusion, we found that persistent MRSA infection was associated with clinical decline in about 50% of patients and that anaerobic conditions, which occur variably in CF airways, affect the expression of virulence factors and antibiotic susceptibility of MRSA more than change in these characteristics over time.
Supplementary Material
HIGHLIGHTS.
Persistent MRSA infection is often associated with clinical decline.
Neither haemolysis nor biofilm formation differ between early to chronic infection
Anaerobic growth conditions alter biofilm formation and haemolysis of MRSA
Anaerobic growth affects antibiotic susceptibility varying by on antibiotic class
Acknowledgements
We are grateful to the UNC Microbiology Laboratory. To Prof. Peter Gilligan, PhD for early insight of storing isolates and to Melissa B. Miller and Melisa Jones for assistance with identification and retrieval of bacterial isolates. We acknowledge the work of Janet Torres Bustos and Gail Carson for their assistance with MRSA identification and laboratory support. We also thank the University of Washington CF Isolate Core (R. Hernandez and J. Burns, directors), S. McNamara and M. Blackledge for identification and provision of isolates and data.
Funding sources:
Cystic Fibrosis Foundation MUHLEB15A0, HOFFMA14A0, BURNS03Y2, DONALD18Y7 and SINGH15R0; NIH K24HL141669, CTSA—UL1TR002489 and P30DK089507.
Abbreviations:
- SA
Staphylococcus aureus
- MRSA
methicillin resistant SA
- UNC
University of NC, Chapel Hill
- UW
Washington Children’s Hospital, Seattle
- FEV1
forced expiratory volume in 1st second
- BMI
Body Mass index
- pvl
Panton-Valentin Leukocidin
- ST
Sequence type
- SCV
small colony variant
- CLSI
Clinical and Laboratory Standards Institute
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- CC
Clonal complex
References
- 1.Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N and Boyle MP, Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA, 2010. 303(23): p. 2386–2392. [DOI] [PubMed] [Google Scholar]
- 2.Ren CL, Morgan WJ, Konstan MW, Schechter MS, Wagener JS, Fisher KA and Regelmann WE, Presence of methicillin resistant Staphylococcus aureus in respiratory cultures from cystic fibrosis patients is associated with lower lung function. Pediatr Pulmonol, 2007. 42(6): p. 513–518. [DOI] [PubMed] [Google Scholar]
- 3.Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ and Goss CH, Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med, 2010. 182(5): p. 627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heltshe SL, Saiman L, Popowitch EB, Miller MB, Kloster M, Thompson V, Ferkol TW, Hoover WC, Schechter MS and Muhlebach MS, Outcomes and Treatment of Chronic Methicillin-Resistant Staphylococcus aureus Differs by Staphylococcal Cassette Chromosome mec (SCCmec) Type in Children With Cystic Fibrosis. J Pediatric Infect Dis Soc, 2015. 4(3): p. 225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esther CR Jr., Muhlebach MS, Ehre C, Hill DB, Wolfgang MC, Kesimer M, Ramsey KA, Markovetz MR, Garbarine IC, Forest MG, et al. , Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis. Sci Transl Med, 2019. 11(486). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowley ES, Kopf SH, LaRiviere A, Ziebis W and Newman DK, Pediatric Cystic Fibrosis Sputum Can Be Chemically Dynamic, Anoxic, and Extremely Reduced Due to Hydrogen Sulfide Formation. MBio, 2015. 6(4): p. e00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sibley CD, Parkins MD, Rabin HR, Duan K, Norgaard JC and Surette MG, A polymicrobial perspective of pulmonary infections exposes an enigmatic pathogen in cystic fibrosis patients. Proc Natl Acad Sci U S A, 2008. 105(39): p. 15070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muhlebach MS, Hatch JE, Einarsson GG, McGrath SJ, Gilipin DF, Lavelle G, Mirkovic B, Murray MA, McNally P, Gotman N, et al. , Anaerobic bacteria cultured from cystic fibrosis airways correlate to milder disease: a multisite study. Eur Respir J, 2018. 52(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akil N and Muhlebach MS, Biology and management of methicillin resistant Staphylococcus aureus in cystic fibrosis. Pediatr Pulmonol, 2018. 53(S3): p. S64–s74. [DOI] [PubMed] [Google Scholar]
- 10.Savage VJ, Chopra I and O’Neill AJ, Population diversification in Staphylococcus aureus biofilms may promote dissemination and persistence. PLoS One, 2013. 8(4): p. e62513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahl B, Herrmann M, Everding AS, Koch HG, Becker K, Harms E, Proctor RA and Peters G, Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J Infect Dis, 1998. 177(4): p. 1023–9. [DOI] [PubMed] [Google Scholar]
- 12.Wolter DJ, Onchiri FM, Emerson J, Precit MR, Lee M, McNamara S, Nay L, Blackledge M, Uluer A, Orenstein DM, et al. , Prevalence and clinical associations of Staphylococcus aureus small-colony variant respiratory infection in children with cystic fibrosis (SCVSA): a multicentre, observational study. Lancet Respir Med, 2019. 7(12): p. 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirschhausen N, Block D, Bianconi I, Bragonzi A, Birtel J, Lee JC, Dubbers A, Kuster P, Kahl J, Peters G, et al. , Extended Staphylococcus aureus persistence in cystic fibrosis is associated with bacterial adaptation. Int J Med Microbiol, 2013. 303(8): p. 685–92. [DOI] [PubMed] [Google Scholar]
- 14.Hankinson JL, Odencrantz JR and Fedan KB, Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med, 1999. 159(1): p. 179–87. [DOI] [PubMed] [Google Scholar]
- 15.Larsen AR, Stegger M and Sorum M, spa typing directly from a mecA, spa and pvl multiplex PCR assay-a cost-effective improvement for methicillin-resistant Staphylococcus aureus surveillance. Clin Microbiol Infect, 2008. 14(6): p. 611–4. [DOI] [PubMed] [Google Scholar]
- 16.Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, Vandenesch F and Etienne J, Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis, 1999. 29(5): p. 1128–32. [DOI] [PubMed] [Google Scholar]
- 17.Shopsin B, Gomez M, Montgomery SO, Smith DH, Waddington M, Dodge DE, Bost DA, Riehman M, Naidich S and Kreiswirth BN, Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol, 1999. 37(11): p. 3556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellmann A, Weniger T, Berssenbrugge C, Rothganger J, Sammeth M, Stoye J and Harmsen D, Based Upon Repeat Pattern (BURP): an algorithm to characterize the long-term evolution of Staphylococcus aureus populations based on spa polymorphisms. BMC Microbiol, 2007. 7: p. 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stepanovic S, Vukovic D, Hola V, Di Bonaventura G, Djukic S, Cirkovic I and Ruzicka F, Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by Staphylococci. APMIS, 2007. 115(8): p. 891–9. [DOI] [PubMed] [Google Scholar]
- 20.Gomes-Fernandes M, Laabei M, Pagan N, Hidalgo J, Molinos S, Villar Hernandez R, Domínguez-Villanueva D, Jenkins ATA, Lacoma A and Prat C, Accessory gene regulator (Agr) functionality in Staphylococcus aureus derived from lower respiratory tract infections. PLoS One, 2017. 12(4): p. e0175552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee TW, Brownlee KG, Conway SP, Denton M and Littlewood JM, Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros, 2003. 2(1): p. 29–34. [DOI] [PubMed] [Google Scholar]
- 22.Tenover FC and Goering RV, Methicillin-resistant Staphylococcus aureus strain USA300: origin and epidemiology. J Antimicrob Chemother, 2009. 64(3): p. 441–6. [DOI] [PubMed] [Google Scholar]
- 23.Champion EA, Miller MB, Popowitch EB, Hobbs MM, Saiman L, Muhlebach MS and STAR-CF., Antimicrobial susceptibility and molecular typing of MRSA in cystic fibrosis. Pediatr Pulmonol, 2013. 49(3): p. 230–237. [DOI] [PubMed] [Google Scholar]
- 24.Harrell LJ and Evans JB, Effect of anaerobiosis on antimicrobial susceptibility of staphylococci. Antimicrob Agents Chemother, 1977. 11(6): p. 1077–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waters VJ, Kidd TJ, Canton R, Ekkelenkamp MB, Johansen HK, LiPuma JJ, Bell SC, Elborn JS, Flume PA, VanDevanter DR, et al. , Reconciling Antimicrobial Susceptibility Testing and Clinical Response in Antimicrobial Treatment of Chronic Cystic Fibrosis Lung Infections. Clin Infect Dis, 2019. 69(10): p. 1812–1816. [DOI] [PubMed] [Google Scholar]
- 26.Lo DK, Muhlebach MS and Smyth AR, Interventions for the eradication of meticillin-resistant Staphylococcus aureus (MRSA) in people with cystic fibrosis. Cochrane Database Syst Rev, 2018. 7: p. CD009650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabryszewski SJ, Wong Fok Lung T, Annavajhala MK, Tomlinson KL, Riquelme SA, Khan IN, Noguera LP, Wickersham M, Zhao A, Mulenos AM, et al. , Metabolic Adaptation in Methicillin-Resistant Staphylococcus aureus Pneumonia. Am J Respir Cell Mol Biol, 2019. 61(2): p. 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan X, Coureuil M, Ramond E, Euphrasie D, Dupuis M, Tros F, Meyer J, Nemazanyy I, Chhuon C, Guerrera IC, et al. , Chronic Staphylococcus aureus Lung Infection Correlates With Proteogenomic and Metabolic Adaptations Leading to an Increased Intracellular Persistence. Clin Infect Dis, 2019. 69(11): p. 1937–1945. [DOI] [PubMed] [Google Scholar]
- 29.Pragman AA, Yarwood JM, Tripp TJ and Schlievert PM, Characterization of virulence factor regulation by SrrAB, a two-component system in Staphylococcus aureus. J Bacteriol, 2004. 186(8): p. 2430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuchs S, Pane-Farre J, Kohler C, Hecker M and Engelmann S, Anaerobic gene expression in Staphylococcus aureus. J Bacteriol, 2007. 189(11): p. 4275–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller M, Gilligan PH, Laboratory aspects of management of chronic pulmonary infections in patients with cystic fibrosis. J Clin Microbiol, 2003. 41(9): p. 4009–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goerke C, Gressinger M, Endler K, Breitkopf C, Wardecki K, Stern M, Wolz C and Kahl BC, High phenotypic diversity in infecting but not in colonizing Staphylococcus aureus populations. Environ Microbiol, 2007. 9(12): p. 3134–42. [DOI] [PubMed] [Google Scholar]
- 33.Al-Zubeidi D, Hogan PG, Boyle M, Burnham CA and Fritz SA, Molecular epidemiology of methicillin-resistant Staphylococcus aureus isolated in serial cultures from the respiratory tract of children with cystic fibrosis. Pediatr Infect Dis J, 2014. 33(6): p. 549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahl BC, Duebbers A, Lubritz G, Haeberle J, Koch HG, Ritzerfeld B, Reilly M, Harms E, Proctor RA, Herrmann M, et al. , Characteristics of Staphylococcus aureus, isolated from airways of cystic fibrosis patients, and their small colony variants. J Clin Microbiol, 2003. 41(9): p. 4424–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azarian T, Ridgway JP, Yin Z and David MZ, Long-Term Intrahost Evolution of Methicillin Resistant Staphylococcus aureus Among Cystic Fibrosis Patients With Respiratory Carriage. Front Genet, 2019. 10: p. 546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodrich JS, Sutton-Shields TN, Kerr A, Wedd JP, Miller MB and Gilligan PH, Prevalence of community-associated methicillin-resistant Staphylococcus aureus in patients with cystic fibrosis. J Clin Microbiol, 2009. 47(4): p. 1231–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


