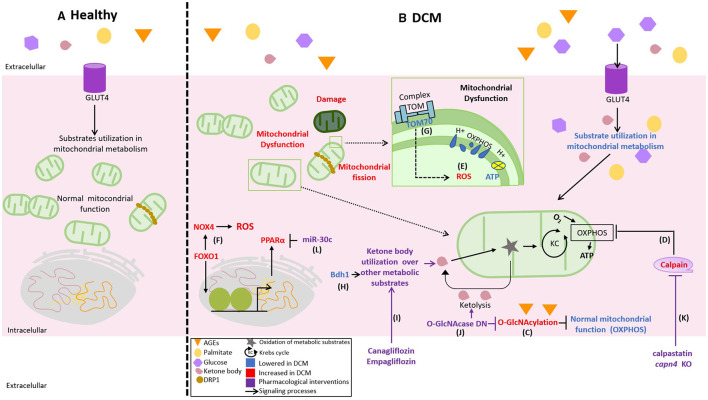
Figure 2.
Novel differences between healthy and DCM mitochondrial function and its pharmacological targets. (A) Normally, the mitochondria used glucose, lipids, and ketones as the fuel to obtain adenosine triphosphate (ATP) in the oxidative phosphorylation (OXPHOS) according to the necessities of the cell, (B) but in DCM, there is an excess of available fuels, increasing glucose, palmitate, and advanced glycation end products (AGEs) that affect the flexibility in substrate use by the mitochondria triggering mitochondrial dysfunction. This process is evidenced by a lower mitochondrial membrane potential, decreased production of ATP by OXPHOS, and increased reactive oxygen species (ROS). The mechanisms of mitochondrial deterioration in DCM are illustrated as follows: (C) the decrease in OXPHOS activity by O-GlcNAcylation (OGA), (D) the upregulation of calpains in the diabetic heart, (E) the abnormal production of mitochondrial ROS, (F) the production of cytosolic ROS by an increase in the expression of NADPH oxidases 4 (NOX4) indirectly regulated by FOXO1, and (G) the downregulation of TOM70 in the diabetic heart. Thus, therapeutic strategies aiming to improve a mitochondrial function are represented as follows: (H,I) the overexpression of β-hydroxybutyrate-dehydrogenase (BDH1) and Canagliflozin or Empagliflozin to increase the use of ketones, (J) dominant-negative for OGA (O-GlcNAcase DN), (K) inhibition of calpain activity by calpastatin (endogenous calpain inhibitor) or capn4 knockout, and (L) micro-RNA 30c (miR-30c) reduces the proliferator-activated receptor alpha (PPARα) coactivator PGC-1β.